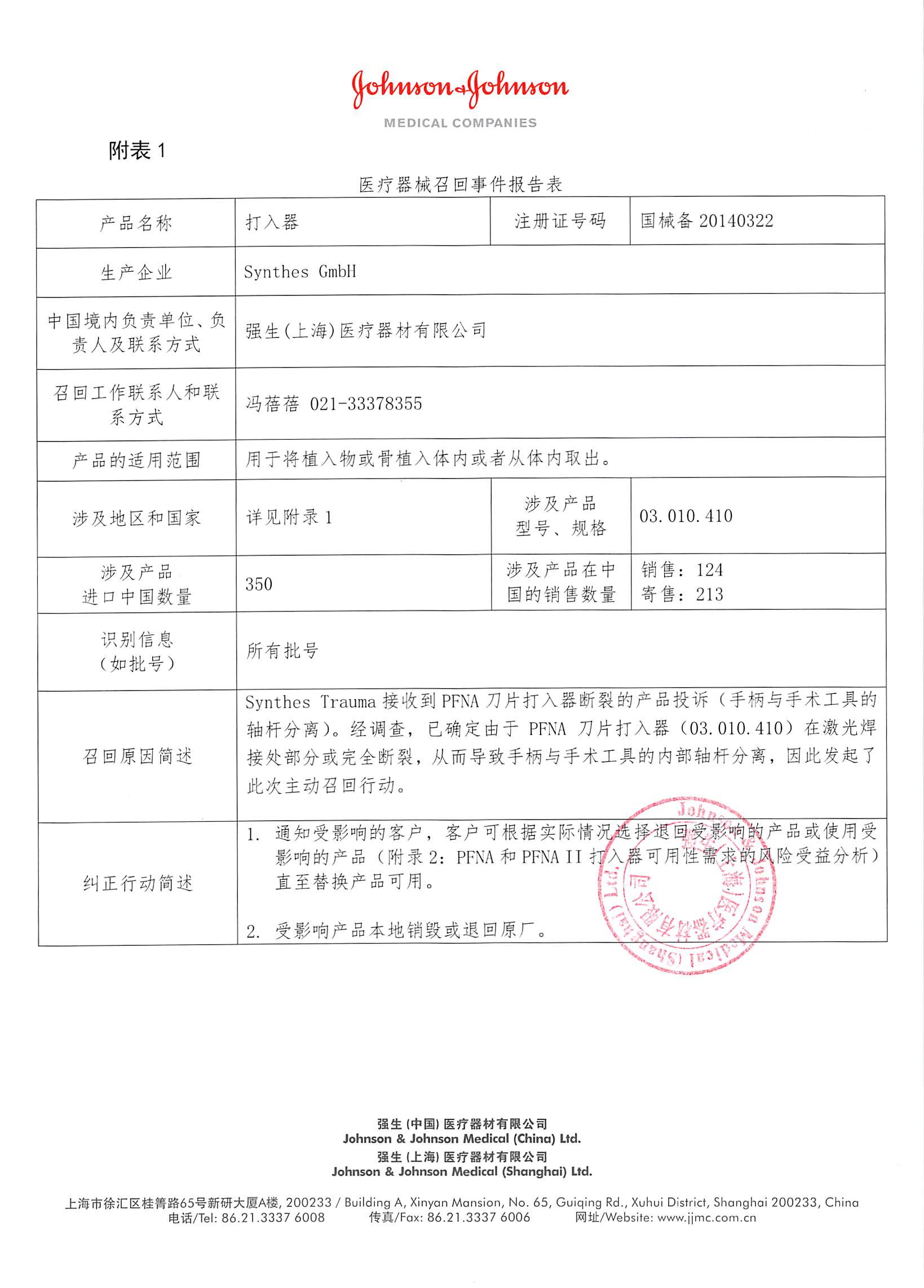
According to the official website of Shanghai Food and Drug Administration on January 13, 2017, Johnson & Johnson (Shanghai) Medical Equipment Co., Ltd. reported: After investigation, it has been determined that the PFNA blade driver is partially or completely broken at the laser welding, resulting in a handle. Separated from the internal shaft of the surgical tool. The company voluntarily recalled related products. The recall level is Level II. Detailed information on the model, specifications and batches of the products can be found in the Medical Device Recall Report Form.

Vats Thoracoscopic Instruments,Thoracoscopic Dissector,Thoracoscopiknot Pushing Stick,Simple Joints Thoracoscopic Instruments
ZHEJIANG SHENDASIAO MEDICAL INSTRUMENT CO.,LTD. , https://www.sdsmedtools.com