Pharmaceutical Network November 23rd Recently, Shanghai Fosun Pharmaceutical Co., Ltd., a subsidiary of Shanghai Fosun Pharmaceutical (Group) Co., Ltd. (hereinafter referred to as "Chongqing Pharmaceutical Friends"), received the Kliner issued by the State Food and Drug Administration. "Pharmaceutical Supplement Application Approval" (Approval No.: 2018B04085, 2018B04086) of themycin capsule, the drug is evaluated by generic consistency.
Basic situation of drugs
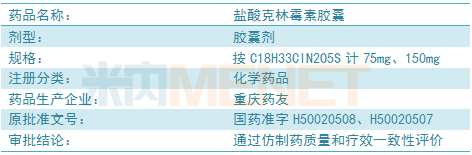
The drug is an anti-infective drug and is mainly used for infections caused by sensitive strains such as Streptococcus, Staphylococcus and Anaerobe.
Sales of clindamycin in Chinese public medical institutions (unit: 10,000 yuan)
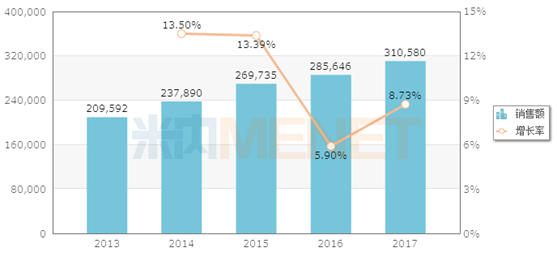
According to the data of Mine, in 2017, the sales of clindamycin in the public hospitals , county-level public hospitals, urban community centers and township health centers (referred to as Chinese public medical institutions) in China reached more than 3 billion yuan. From the perspective of dosage form, clindamycin has injections, tablets, capsules and other dosage forms, of which injections accounted for 69.94% and tablets accounted for 24.67%.
2017 Clindamycin TOP20 brand pattern of public medical institutions in China
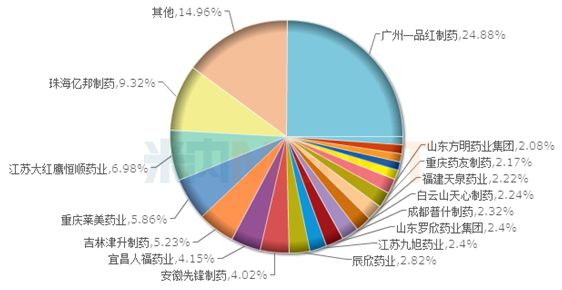
As of the announcement date, clindamycin preparations that have been listed in China include Kelley Lin® of Guangzhou Yipinhong Pharmaceutical Co., Ltd. and Telixian® of Jiangsu Dahongying Hengshun Pharmaceutical Co., Ltd.
As of October 2018, the Group (ie the company and its holding subsidiaries/units) has invested R&D expenses of approximately RMB 6.55 million (unaudited) for the drug conformity assessment at this stage.
Source: Listed company announcement, Minenet database
Hand massager
Hand massager
Shenzhen Jie Zhong Lian Investment Co., Ltd. , https://www.szmeizons.com