In the previous article ( leading to the past, seeing today's OpenSPR, Biacore and Fortebio are weak and weak ), we compared the mainstream unlabeled intermolecular interaction detection technology on the market. Today we will talk about the detection of intermolecular interactions based on fluorescent labels.
Compared to unlabeled techniques, the use of fluorescent techniques to detect intermolecular interactions is less expensive, such as fluorescence resonance energy transfer and gel migration experiments, and can be performed without the need for expensive instrumentation. However, fluorescent label-based detection techniques also have their own limitations, such as gel migration experiments can only be used to detect the interaction between proteins and nucleic acids. So in a specific experiment, how should researchers choose the right detection technology? Don't worry, let's introduce the most common fluorescence binding analysis techniques to give you some ideas.
Fluorescence polarization (FP)
The fluorescence polarization technique utilizes polarized light to excite fluorophores on the probe, and the emitted light is polarized or unpolarized depending on the state of migration of the probe. For example, a biomacromolecule rotates relatively slowly, and a polarized signal is generated when excited by polarized light. Small molecules can depolarize the signal due to faster rotation. By analyzing the intensity of the polarized signal of the emitted light, the intermolecular interaction and enzyme activity detection can be quickly and quantitatively analyzed.
Fluorescence polarization technology is very suitable for high-throughput screening experiments, but the binding kinetic parameters (binding/dissociation rate constant) cannot be obtained, and the fluorophores may affect the intermolecular binding and need to be verified by other techniques. Luteolin inhibits Musashi1 binding to RNA and disrupts cancer phenotypes in glioblastoma cells , a high-throughput screening of small molecule inhibitors targeting cancer-associated proteins, was validated using our LSPR technology.
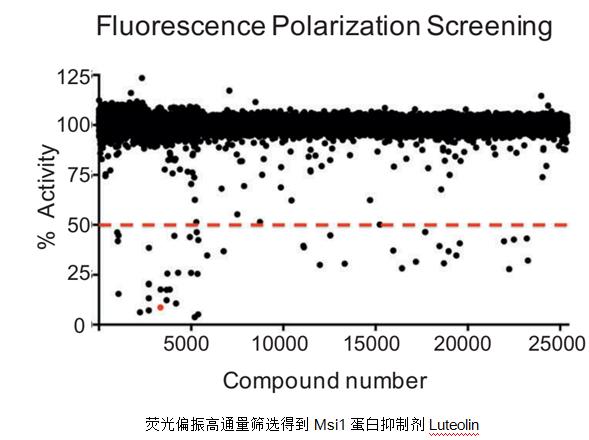

Fluorescence resonance energy transfer (FRET)
Fluorescence resonance energy transfer means that in two different fluorophores, if the emission spectrum of one fluorophore (donor Donor) overlaps with the absorption spectrum of another group (receptor acceptor), when When the distance between the fluorescent groups is appropriate (generally less than 100 Ã…), the phenomenon that the fluorescence energy is transferred from the donor to the receptor can be observed. This allows us to detect interactions between fusion proteins with donor and acceptor groups, respectively. Compared with other technologies, FRET's greatest advantage is that it can be used to study protein-protein interactions under physiological conditions of living cells. However, there are fewer types of samples that can be detected, and it is currently only suitable for the detection of proteins and nucleic acids. Also, due to the involvement of fluorophores, there may be an effect on the interaction between molecules.
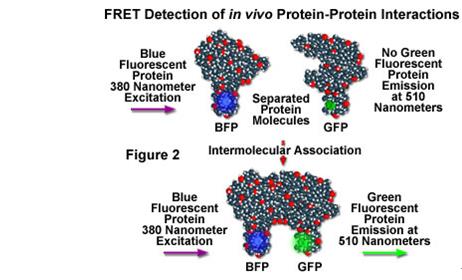
Gel Migration Experiment (EMSA)
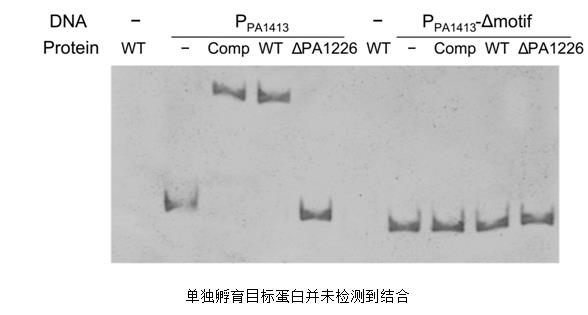
Gel migration experiments, also known as gel retardation experiments or electrophoretic mobility shift assays (EMSA), are techniques for the detection of protein-nucleic acid interactions. Originally used as a confirmatory experiment for the interaction of transcription factors with promoters, it can also be applied in interaction with protein-DNA and protein-RNA. EMSA is based primarily on the principle that protein-probe complexes migrate slowly during gel electrophoresis. According to the experimental design of specific and non-specific probes, when the nucleic acid probe is mixed with the sample protein, the protein and probe complex which can bind to the nucleic acid probe in the sample form a protein-probe complex; this complex has a large molecular weight The migration is slower when performing non-denaturing polyacrylamide gel electrophoresis, but the probe without binding protein is faster; the incubated sample is subjected to polyacrylamide gel electrophoresis and after transfection, if the protein-probe complex Forming a band at the front of the membrane indicates an interaction between the protein and the target probe.
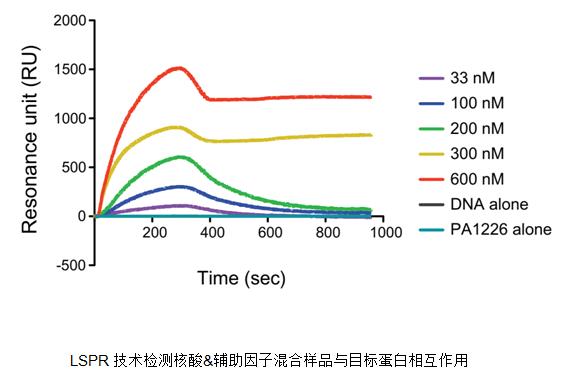
The EMSA technology threshold and experimental cost are relatively low, but it is only suitable for the interaction detection of protein and nucleic acid, and the experimental procedure is relatively cumbersome. In the article "Nove dual regulators of Pseudomonas aeruginosa essential for productive biofilms and virulence, the researchers first used EMSA to detect the interaction between proteins and nucleic acids. After discovering that the interaction between proteins and nucleic acids requires the involvement of helper factors, use us. The LSPR technology was quickly verified.
Micro thermophoresis (MST)
The micro-thermal kinetic technique uses infrared laser heating to generate a microscopic temperature gradient field, which leads to molecular directional movement, and then monitors the fluorescence signal changes in the observed area by covalently binding fluorescent dye or tryptophan autofluorescence to calculate two Constants such as KD between interacting molecules. MST technology does not require surface fixation, and can detect any interaction between molecules such as biomolecules, compounds, nanomaterials, etc. in solution, and has a wide range of applications. However, binding kinetic parameters (binding/dissociation rate constants) are not available, and covalently bound dyes may have an effect on the interaction between molecules.
In summary, compared with the unlabeled intermolecular interaction detection technology, the fluorescence-based intermolecular interaction detection technology has the following in common:
Unable to provide binding kinetic parameters (binding/dissociation rate constant)
Fluorescent groups may affect the intermolecular binding
Usually need to use other technology to verify
Surface Plasmon Resonance (SPR) is the gold standard for intermolecular interaction detection, eliminating the need to label biomolecules and making the data more accurate. In addition to the two intermolecular affinities, it also provides binding kinetic parameters (binding/dissociation rate constants) to help researchers understand the biological binding process and the mechanism of drug action. Canada's Nicoya's OpenSPR greatly reduces the operational difficulty of traditional SPR instruments, while using a simple and stable optical system and large aperture flow path, allowing users to stay away from high maintenance costs.
Advantages of Nicoya OpenSPR molecular interaction instrument in research

Multi-parameter detection---Ka, Kd, ​​KD, EC50, protein concentration determination
Innovative LSPR technology --- detection is not affected by temperature and buffer refractive index
Easy to operate - 1-2 hours to quickly master
Wide range detection --- pM-mM
Low cost - real-time mark-free, chip recyclable
Video Doorbell,Intercom Video Doorbell,Sd Card Video Doorbell,Camera Doorbell
Shenzhen Zuomi Technology Co., Ltd. , https://www.bkvis.com