Two-pronged treatment of advanced melanoma, immunotherapy welcomes new combination
June 11, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Recently, Syndax and Nektar announced a clinical collaboration to evaluate the safety of Nektar NKTR-214 in combination with entinostat in Syndax for the treatment of patients with metastatic melanoma who have received anti-PD-1 (programmed death receptor-1) drugs. Sex and effectiveness.
Syndax, based in Massachusetts, USA, is a biopharmaceutical company that develops a range of innovative cancer treatments in clinical and preclinical stages. San Francisco-based Nektar Therapeutics Biopharmaceuticals has a strong drug development pipeline in the fields of oncology, immunology and pain, as well as a range of approved co-pharmaceuticals.

Entinostat is Syndax's oral small molecule class 1 specific HDAC inhibitor. In preclinical trials, the combination of entinostat and NKTR-214 significantly inhibited tumor growth in kidney and colon cancer models. The anti-tumor activity of this combination increases dramatically with the activation and cytotoxic activity of CD8+ T cells in tumors, as well as by immunosuppressive cells in the tumor microenvironment. This work was recently published in the 2018 American Association of Cancer Research Annual Meeting. In addition, entinostat is currently being used in phase 3 clinical trials with exemestane to treat hormone receptor-positive, human epidermal growth factor receptor 2 negative advanced breast cancer, and has been awarded a breakthrough therapy by the FDA.
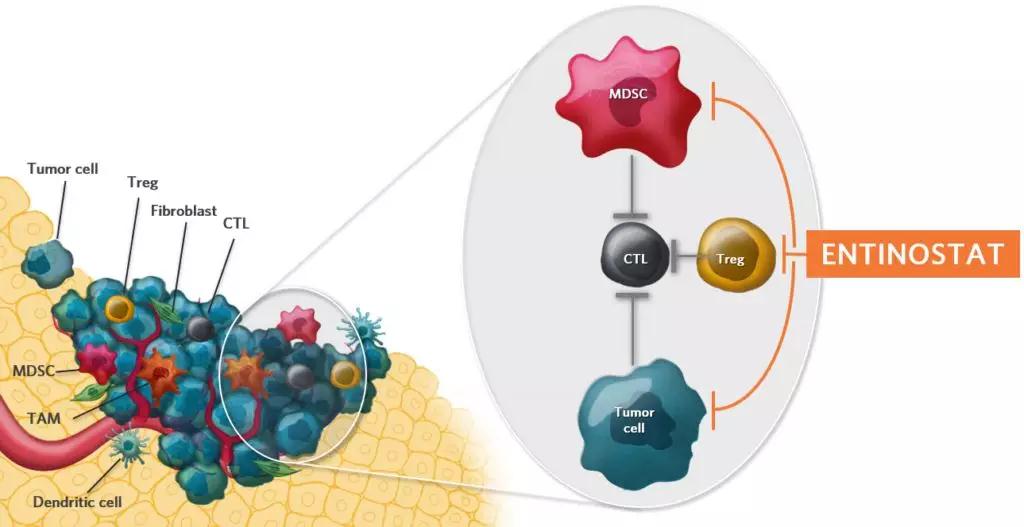
â–² The mechanism of action of Entinostat (Source: Syndax official website)
NKTR-214 is a CD122-preferred agonist under development designed to stimulate anti-cancer immune cells in vivo by targeting CD122-specific receptors on the surface of CD8+ effector T cells and natural killer cells. The in vivo culture of these tumor infiltrating lymphocytes (TILs) to supplement the immune system is critical for many patients, especially those who lack adequate TIL and are not eligible for checkpoint inhibitor therapy. In preclinical studies, NKTR-214 caused these cells to rapidly expand and mobilize into the tumor microenvironment. It has an antibody-like dosing regimen similar to existing approved checkpoint inhibitors.
Under the terms of the agreement, Syndax and Nektar will collaborate on a study to evaluate this combination. The Phase 1b portion of the trial is intended to determine the safety and recommended dosage of the combined dosing regimen. Phase 2 clinical trials were designed to assess efficacy, such as objective response rate and duration of remission, as well as progression-free survival (PFS) and overall survival (OS). The patient's relevant biomarkers will be included in the analysis, including the exploratory analysis of the potential for elevated levels of classical peripheral blood mononuclear cells. Syndax will be responsible for the Phase 1b / 2 trial, and one of the provisions of the agreement includes the possibility of expanding the scope of cooperation between the two parties and conducting key trials based on common interests.
Syndax CEO Dr. Briggs W. Morrison said: "We are pleased to work with Nektar to establish a clinical collaboration strategy to test the novel combination of entinostat and cutting-edge immunotherapy. Use entinostat and high-dose IL-2 in renal cell carcinoma Phase 2 clinical data and our promising preclinical data using NKTR-214 laid the scientific and clinical foundation for this collaboration, and working with Nektar enabled us to perform PD-1 refractory metastatic melanoma patients Effective treatment also complements our exciting data on Keytruda using entinostat in a similar population."

â–² Dr. Jonathan Zalevsky (Source: Nektar)
Dr. Jonathan Zalevsky, Senior Vice President and Chief Scientific Officer of Nektar, said: "The combination of NKTR-214 and entinostat demonstrates that our preclinical models have unique synergies that require further clinical research. Importantly, we observed a combination After treatment, the level of cytokine-positive tumor infiltrating T cells is elevated, and we believe this important preclinical finding can be translated into remission in patients who are difficult to cure after a checkpoint inhibitor. We look forward to working with Syndax Cooperation brings this combination to the clinic."
We hope that this collaboration will bring us new anti-cancer combination therapies that will bring relief to patients with multiple types of cancer.
Reference materials:
[1] Syndax and Nektar Therapeutics Announce Immuno-Oncology Clinical Trial Collaboration
[2] Syndax official website
Sanitary Valve,Angle Seat Valve,Sampling Valve,Pneumatic Diaphragm Valve
Wenzhou Gaoya Light Industry Machinery Co.,ltd. , https://www.hongyafitting.com