Medical Network April 19th, the latest report released by Kerui Wei'an, "The Most Notable Drug Forecast 2018" predicts 12 new drugs that have been listed in 2018 (listed or possibly listed). These new drugs are within 5 years. Sales will reach the blockbuster level.
More than half of the 12 drugs were evaluated by priority review, breakthrough therapy or fast-track, 5 of which were first-inclass new drugs, 4 were orphan drugs, and only 1 anti-cancer drug On the list, that is, Johnson's Erleada (apalutamide).
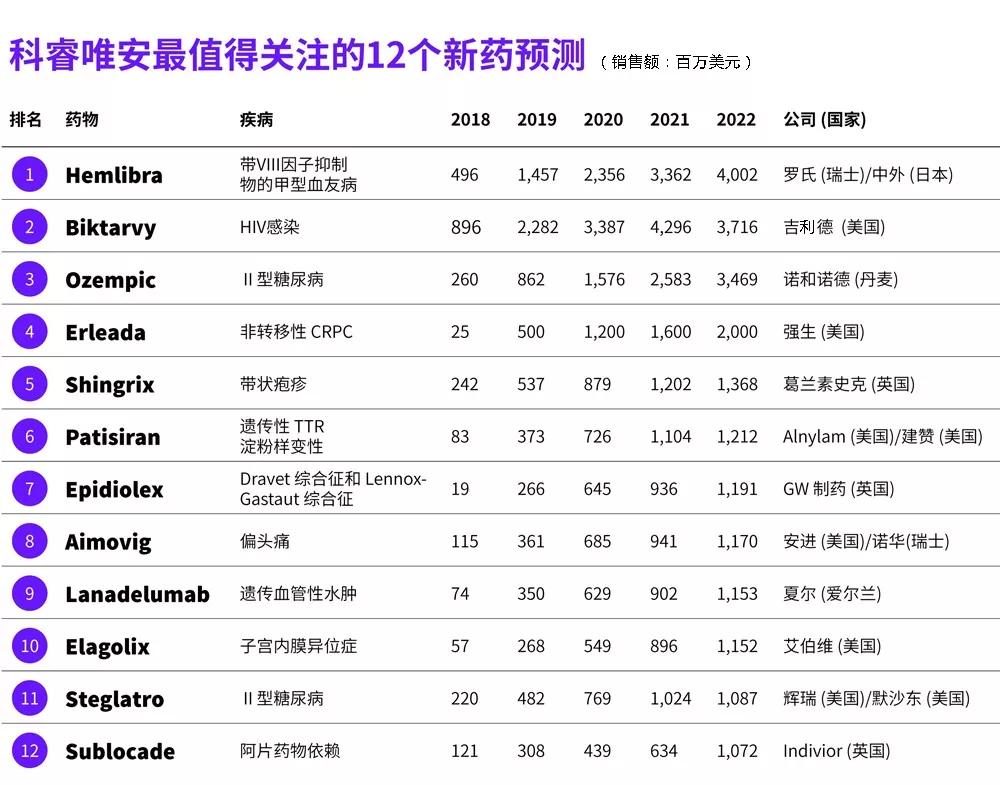
1 Hemlibra
Future leader in the hemophilia market
The appearance of factor VIII inhibitors is the most serious and most challenging complication in the treatment of hemophilia. Hemlibra has been approved for the prevention of hemorrhagic hemophilia A in children or adults with factor VIII inhibitors to prevent or reduce the occurrence of bleeding events. The drug's approval for the disease has brought the first new treatment in 20 years. Other drugs available in the field are mainly Shire's Feiba (prothrombin complex) and Norderold's NovoSeven. (recombinant factor VIIa).
In fact, the FDA's decision to approve Hemlibra was February 2018, but the FDA approved Hemlibra in advance in November 2017 for priority review, breakthrough therapy, and orphan drug certification.
In any case, Hemlibra is one of the most noteworthy new drugs in 2018, as the sales promotion of the product will be fully rolled out in 2018 and will be approved in more countries. In February 2018, the European Medicines Agency (EMA) approved Hemlibra, which is expected to land in both the EU and Japan markets during the year. It is expected to have sales of US$496 million in 2018 and US$1.457 billion in 2019. Up to $4.02 billion.
After 2018, Feiba and NovoSeven may experience a sharp decline in sales due to Hemlibra's competition. The biggest impact is that Hemlibra will be approved for hemophilia A, which does not limit Factor VIII inhibitors, which will greatly broaden its range of medications.
Hemlibra's new mechanism of action, superior efficacy and potential frequency of administration per month will have a huge impact on the VIII factor market. Although there is a black box warning (the FDA has a serious warning of potentially serious adverse reactions ) the risk of thrombosis, Hemlibra will still lead the hemophilia treatment market beyond 2020.
2 Biktarvy
Grab the HIV market with GSK products
Gilead's daily HIV combination therapy Biktarvy is a combination of the new integrase inhibitor bictegravir, the nucleoside reverse transcriptase inhibitor tenofovir alafenamide and emtricitabine, in 2018 2 Approved in the US in the middle of the month and quickly listed.
Gilead and GSK competed fiercely in the anti-HIV market. In 2014, GSK launched Triumeq, the success of which enabled it to regain a game in the anti-HIV market. In response, Gilead has introduced several best-in-class combination solutions, including Genvoya, which went on sale in 2015, and Odefsey and Descovy, which launched in 2016, which helped Gilead regain lost market share.
In January 2018, GSK also launched Drututwei/Ripvirin, an optimal combination of integrase inhibitor Tivicay and rivimivir. The safety and efficacy of bictegravir is comparable to that of Tivicay, and, like Tivicay, is a true once-integration inhibitor, without the need for any additional enhancer.
With the launch of tenofovir disoproxil in December 2017, Atripla and Truvada generics are also expected to be available in the near future, and GSK's market share based on Tivicay therapy continues to increase, and Gilead is likely to be fully operational. Promote Biktarvy.
Biktarvy is expected to reach $896 million in sales in 2018, and will rise rapidly to $2.282 billion in 2019 and rise to $3.716 billion in 2022.
3 Ozempic
Competitiveness to be fully explored
Novo Nordisk GLP-1 analogue Ozempic (somaglutide), which was administered once a week, was approved for the treatment of type 2 diabetes in December 2017 and was launched in February 2018.
The market share of Novo (liuraglutide) administered once a day is gradually losing, and the market share of GLP-1 analogues administered once a week is gradually increasing, especially the courtesy of Tulipan ( Degree glycopeptide). However, data from the SUSTAIN 7 published in 2017 shows that Ozempic's hypoglycemic effect and weight loss are better than Truecity.
REWIND, a clinical trial that collects evidence of cardiovascular benefits from Trulicity, is still underway and data is expected to be published in 2018. Because Ozempic has obtained positive evidence of cardiovascular benefits, it will force Trulicity to help Novo Nordisk grab the GLP-1 market share. Despite this, Trulicity will remain the leader in the G22-1 market in 2022, with sales forecasting at $4.009 billion, Novo Force at $3.912 billion, and Ozempic at $3.469 billion.
Injectable GLP-1 analogues are usually used under conditions in which oral hypoglycemic agents are unable to control blood glucose, whereas once-daily oral somaglutide (in the PIONEER project evaluation) will change the "rules of the game for type 2 diabetes treatment". ". Oral GLP-1 replaces small molecule hypoglycemic agents at an earlier stage of treatment, which is not only more effective in reducing blood sugar, but also benefits cardiovascular. In addition, somaglutide is also conducting clinical studies of obesity and clinical studies of nonalcoholic fatty liver disease (NASH), which will pose a comprehensive threat to competing products.
4 Erleada
First mover advantage drives sales growth
The castration-resistant prostate cancer (CRPC) market is dominated by Johnson & Johnson's new generation of oral anti-androgen drugs Zytiga (Abiton) and Anstellai/Pfizer's Xtandi (Enzalamide), however both products are only Batch used for transferable CRPC. Erleada, a second-generation anti-androgen drug approved in mid-February, in 2018, took the lead and became the first treatment in the field of non-metastatic CRPC.
Xtandi has shown positive data in the clinical trial of non-metastatic CRPC (PROSPER) and has submitted a listing application for this indication to the FDA. Despite this, Erleada has a first-mover advantage, and the product entered the US market shortly after it was approved, and its sales are expected to reach the blockbuster level by 2020.
In addition to non-metastatic CRPC, Erleada has the potential to be approved for other indications, including metastatic hormone-sensitive prostate cancer (TITAN trial), local high-risk/local advanced prostate cancer (ATLAS trial), and combined abiraterone treatment without chemotherapy. Sex CRPC, these will become the driving force for its rapid growth in sales.
5 Shingrix
Two years or can catch up with the competing products
After obtaining FDA approval in October 2017, GSK's Shingrix (recombinant herpes zoster vaccine, adjuvant) will be launched in early 2018. It is the first new herpes zoster vaccine in more than a decade, and Merck's Zostavax is the only FDA approved vaccine for herpes zoster.
Zostavax's preventive effects will gradually decline over time, while Shingrix continues to provide protection and is still effective against Zostavax's prevention of failure. Although Zostavax requires only one dose and Shingrix requires two doses, the US Centers for Disease Control and Prevention has officially recommended the use of Shingrix for vaccination in people over 50 years of age in January 2018.
Within two years of launch, Shingrix is ​​expected to replace Zostavax as a leader in the herpes zoster vaccine market, with Shingrix expected sales of $537 million in 2019, while Zostavax sales will fall from $765 million (peak) in 2014 to 4.92. One hundred million U.S. dollars. In addition, Shinglix's sales will exceed $1 billion by 2021.
6 Patisiran
Expected to become the first RNAi drug in the United States
The indication for Patisiran's application is hereditary transthyretin-associated amyloidosis, which, if successfully approved, will become the first RNA interference drug (RNAi) in the United States. Prior to the FDA has granted patisiran priority review, fast track and breakthrough therapy identification, PDUFA deadline is August 2018. In Europe, EMA has granted patisiran an accelerated review qualification. The product is expected to land in the United States and Europe at the end of 2018, becoming the standard treatment for hereditary transthyretin-related amyloidosis.
The current standard treatment for this disease is liver transplantation, but this treatment has the risk of surgery, acute liver rejection, infection risk, high clinical requirements, and can only be treated in patients who can receive intervention.
Pfizer's Vyndaqel (tafamidis) is the first anti-amyloid degeneration drug, but is currently only approved for familial amyloid polyneuropathy. Not only that, but the product is limited to early use of the disease, and the United States has not yet been approved for marketing.
7 Epidiolex
Build cannabis extract medical template
GW Pharmaceutical's Epidiolex (plant-derived cannabidiol) is expected to be the first antiepileptic drug containing cannabidiol. The FDA has granted it priority review. The PDUFA period is June 2018.
In addition, a European listing application (MAA) has also been submitted. Because Epidiolex is very convincing for the efficacy data of Dravet syndrome and Lennox-Gastaut, the probability of approval is high.
Although Epidiolex may be approved for listing, there are still policy barriers to sales. This barrier limits the use of all cannabis extracts, especially in the United States, and has strict guidelines governing the use of cannabis drugs.
Still, by 2022, Epidiolex's annual sales are expected to reach $1.191 billion. Epidiolex is well worth looking forward to, paving the way for the medical use of cannabis extract.
8 Aimovig
The most important time to market
Aimovig (erenumab) is likely to be the first CGRP inhibitor to be marketed. Getting the first listing right is crucial for Aimovig because the competition for two monoclonal antibodies from the same target is coming. Lilly's galcanezumab (1 per month) and Teva's fremanezumab (1 per month or once every 3 months) have submitted a listing application to FDA. Alder's eptinezumab (1 every 3 months) is still undergoing Phase III clinical trials, but with a response rate of 100% in some patients, the product is expected to be submitted for marketing in 2018.
Aimovig is expected to be the “leader†of the CGRP inhibitor market with a projected sales of $1.17 billion in 2022; fremanezumab ranks second with a one-pin advantage in March, with sales forecasting $9.99 million; galcanezumab ranked third Position, forecast sales of $ 546 million; followed by eptinezumab, forecast sales of $ 368 million. Due to strong clinical data, eptinezumab's sales will rise at a high rate, expected to reach 946 million US dollars in 2023.
It is worth noting that there will be competition for oral CGRP inhibitors in the future, such as the atogepant of Aerjian and the rimegepant of Biohaven, which will take a big share of the market because of the advantages of oral administration.
9 Lanadelumab
More competitive advantages help sales
The application for the listing of the new hereditary angioedema drug lanadelumab has been submitted and has been awarded the US priority review and the accelerated assessment of the European Union.
Charles's Cinryze (human complement C1 esterase inhibitor) is currently the main product to prevent the occurrence of angioedema, but the main drawback is the need for intravenous administration. The first subcutaneous drug product was the complement C1 esterase inhibitor Haegarda, which was approved by CLS Bellin in 2017. Although the drug has a first-mover advantage, lanadelumab will still dominate the first-line prevention market for hereditary angioedema, and because The curative effect is superior and the administration is convenient, and the dosage is bound to increase substantially.
Although Haegarda and Cinryze are also two-week treatments, lanadelumab is expected to be administered once a month on the basis of two weeks of administration. At the same time, Lanadelumab has less injectable doses than Haegarda and requires less time to inject, all of which will give it a competitive edge to generate huge sales.
Cinryze's sales in 2018 are expected to be $665 million, but will fall to $283 million in 2022, while lanadelumab's sales will increase from $74 million in 2018 to $1.153 billion in 2022.
10 Elagolix
Fill the blank market
Abravi's Elagolix has filed a market application for painful endometriosis with the FDA, and the FDA has granted the drug a priority review. However, on April 10, Aibowei announced that it had received a notice. The FDA requested an extension of the review time to fully evaluate the data on the effects of drugs on liver function in the Elagolix listing application. The PDUFA is scheduled to be extended to the third quarter of this year.
Endometriosis is an incurable disease that can only be symptomatic and is a lifelong treatment. Common treatment options include oral contraceptives, progesterone, danazol, opioids and gonadotropin-releasing hormone ( GnRH) agonist (Lupron, Aberweier's leuprolide injection microspheres) and the like. Many of these therapies are super-instructive medications, which are inconvenient to administer and have many adverse effects such as weight gain, bone loss and menopausal side effects.
Elagolix is ​​a pioneering oral GnRH antagonist, because 170 million women worldwide are affected by endometriosis, so even a slight market penetration can bring huge sales. But in the next few years, Elagolix will face the competition of Myovant's relugolix, an oral GnRH antagonist in Phase III clinical development, and is expected to reach the blockbuster level in 2024.
11 Steglatro
SGLT-2 inhibitor market breakout
Direct competitors to the SGLT-2 inhibitor Steglatro (ertugliflozin) include Johnson & Johnson's Invokana (Cagrequin, marketed in 2013), AstraZeneca's Farxiga (Dagrenet, initial listing in 2014) and Boehringer Ingelheim's Jardiance (Engeleq, listed at the end of 2014).
Steglatro has a unique advantage that helps it grab market share in the fierce competition. The clinical trials of the combination with metformin alone and in combination with sitagliptin have demonstrated the effectiveness of Steglatro, and Steglatro has been shown to significantly reduce body weight and blood pressure in all clinical trials. This prompted the FDA to approve Steglatro monotherapy, combined with sitagliptin (Steglujan) and metformin (Seglurome) in 2017.
A clinical study (VERTIS CV) that collects evidence of Stelantro's cardiovascular benefit is still underway and results are expected to be published in 2019. Steglatro's positive cardiovascular benefit data will be key to consolidating its position in SLGT-2 inhibitors.
In 2016, Invokana was the only blockbuster of SGLT-2 inhibitors with sales of $1,407 million. Cardiovascular benefits, as well as other advantages, will help sales of these drugs rise at a high rate. By 2022, Farxiga is expected to be the "leader" of the SGLT-2 inhibitor market (predicted sales of $2.025 billion), and Jardiance will take the second place (predicted sales of $1.713 billion), Lexicon/Sinofi The SGLT-1/2 dual inhibitor sotagliflozin will be ranked third (predicted sales of $1.193 billion). Sotagliflozin is likely to submit a listing application in the first half of 2018 and is expected to enter the market in 2019. This will be followed by Steglatro and Invokana, with projected sales of $1,087 million and $652 million in 2022, respectively. Invokana was given a black box warning due to the observed risk of amputation in the CANVAS trial, which would result in a decline in sales.
12 Sublocade
Make up for current treatment deficiencies
Indivior's Sublocade (monthly buprenorphine injection) received FDA approval in November 2017, the first monthly buprenorphine prescription.
The drug-assisted withdrawal treatment market will change dramatically as Sublocade goes public in March 2018. The current drug-assisted withdrawal treatment products have many limitations, such as the treatment burden and patient compliance problems of oral therapy once a day, the surgical conditions of implants and the risk of surgery, and Vivitrol has a withdrawal period. The problem of limitations, and these problems just leave room for new treatments.
Sublocade only needs to receive subcutaneous injections at medical institutions once a month. There is no withdrawal period requirement, and many problems in current therapy are well solved.
Considering the serious crisis of opioid abuse in the United States, Sublocade's sales are expected to reach the blockbuster level, with projected sales of $1,072 million in 2022.
GMP ATS Injection, Tetanus Antitoxin, Tetanus Toxoid ,Tetanus Antitoxin Injection, Antitetanus, Refined Tetanus Antitoxinsupplier in China
Tetanus Antitoxin,Tetanus Toxoid,Tetanus Antitoxin Injection,Antitetanus&Refined Tetanus Antitoxin
FOSHAN PHARMA CO., LTD. , https://www.full-pharma.com