It was learned that on November 16, 2018, the Medical Device China Testing Center of WuXi PharmaTech Testing Division was officially launched and launched in Suzhou, Jiangsu Province. This marks the full implementation of WuXi PharmaTech's medical device testing business in the Chinese market, and works with existing US testing centers to form a global integrated medical device testing platform. In the future, the company will focus on providing one-stop services from research and development to testing to production and product launch, build international communication channels, accelerate the entry of international medical device products into China, and empower Chinese medical device companies to promote the development of global health industry.
Deputy Secretary of Wuzhong District Party Committee of Suzhou City, Ms. Chen Yu, Mayor of Wuzhong District People's Government, Member of the Standing Committee of Wuzhong District Committee of Suzhou City, Deputy Secretary of the Party Working Committee of Wuzhong Economic and Technological Development Zone, and Deputy Director of the Management Committee Mr. Gu Yuqi, Wu Mr. Xu Xing, Member of the Standing Committee of the Central Committee, Deputy Director of the Wuzhong District People's Government, Mr. Zhang Lin, Deputy Secretary General of China Medical Device Industry Association, Dr. Yang Qing, Executive Vice President and Chief Commercial Officer of WuXi PharmaTech, Mr. Tong Guodong, Chief Operating Officer of WuXi PharmaTech Dr. Liu Busan, Senior Vice President and Chief Operating Officer of WuXi PharmaTech, delivered a speech and jointly cut the ribbon for the completion of the center.

With the development of China's economy, the aging of society, and the increasing incidence of major diseases such as cancer, the domestic medical device industry has developed rapidly. The state has also introduced a series of favorable policies to lay the foundation for future industrial take-off. Industrial development faces enormous challenges at the same time. A large number of new biomaterials are used, and new material composition analysis brings new challenges to the testing field. Many medical devices require both active and passive detection, while domestic expertise in passive detection, especially animal experiments, is relatively scarce. Domestic doctors' demand for specialized and systematic training of medical device testing will grow rapidly in the future, but there is a serious shortage of high-level training institutions. With the continuous improvement of product technology, the demand for domestic medical device manufacturers to enter the European and American markets has increased, and the demand for high-quality, internationally-adapted testing and regulatory services is growing.
“The WuXi PharmaTech test platform hopes to provide an international level of integrated testing solutions to help scientists turn their ideas into medical health products, truly bringing science and technology from the experimental research and development field to the clinic, benefiting the majority of patients.†Dr. Jun said. By providing one-stop medical device testing and regulatory services from research and development to marketization, WuXi PharmaTech Testing Division helps companies to control quality in multiple stages, enabling companies to focus on innovative research and development of medical devices and reduce waste of funds. Realize the empowerment of SMEs.
The completed test center covers an area of ​​15,000 square meters. Through the comprehensive introduction of the capabilities, operation and quality management system of WuXi PharmaTech's US Instrument Testing Center, the center will provide comprehensive medical device testing services in line with international quality standards. Biomaterial analysis toxicology, biocompatibility, risk assessment, product aseptic design microbiology, physical testing of packaging and shelf life, and product batch release testing. At the same time, the center also plans to establish an international level of doctor training center to achieve a close integration of production, education and research to help the steady development of China's medical device industry. In the future, it will provide customers with high standards and high quality testing and related services in the spirit of seeking truth and truth, helping to bring international leading medical and health products to patients around the world and contributing to the growth of China's medical device industry.
Glow In Dark Tape
1. Material and classification of glow in dark tape
We have PET material glow in dark tape and PVC material glow in dark tape. PET material is cheaper but not printable. PVC material support customized printing with low MOQ.
For both materials, we have different kinds according to the glowing time it can last after full charging (0.5hours charging at least). We have 2hours, 4hour, 6hours, 8hours and 10hours according to the glowing period it can last.
2. Colors for choosing
We have light green, pink, orange, red, blue, white for choosing.
3. Features
a. Good ahesion, we use solvent acrylic adhesive, the adhesive is strong and long lasting.
b. Waterproof. Both PET and PVC material are waterproof. Can used for both indoor and outdoor usage.
c. Long service life: 2~3 years even for outdoor usage.
d. Different sizes for choosing: 1.24m x 45.7m log roll, or other customized sizes such as 25mm/ 50mm x 5meters/ 10yards/ 10meters/ 18meters, etc.
e, Accept die cuting to small pieces such as dots, stars, arrows, etc,







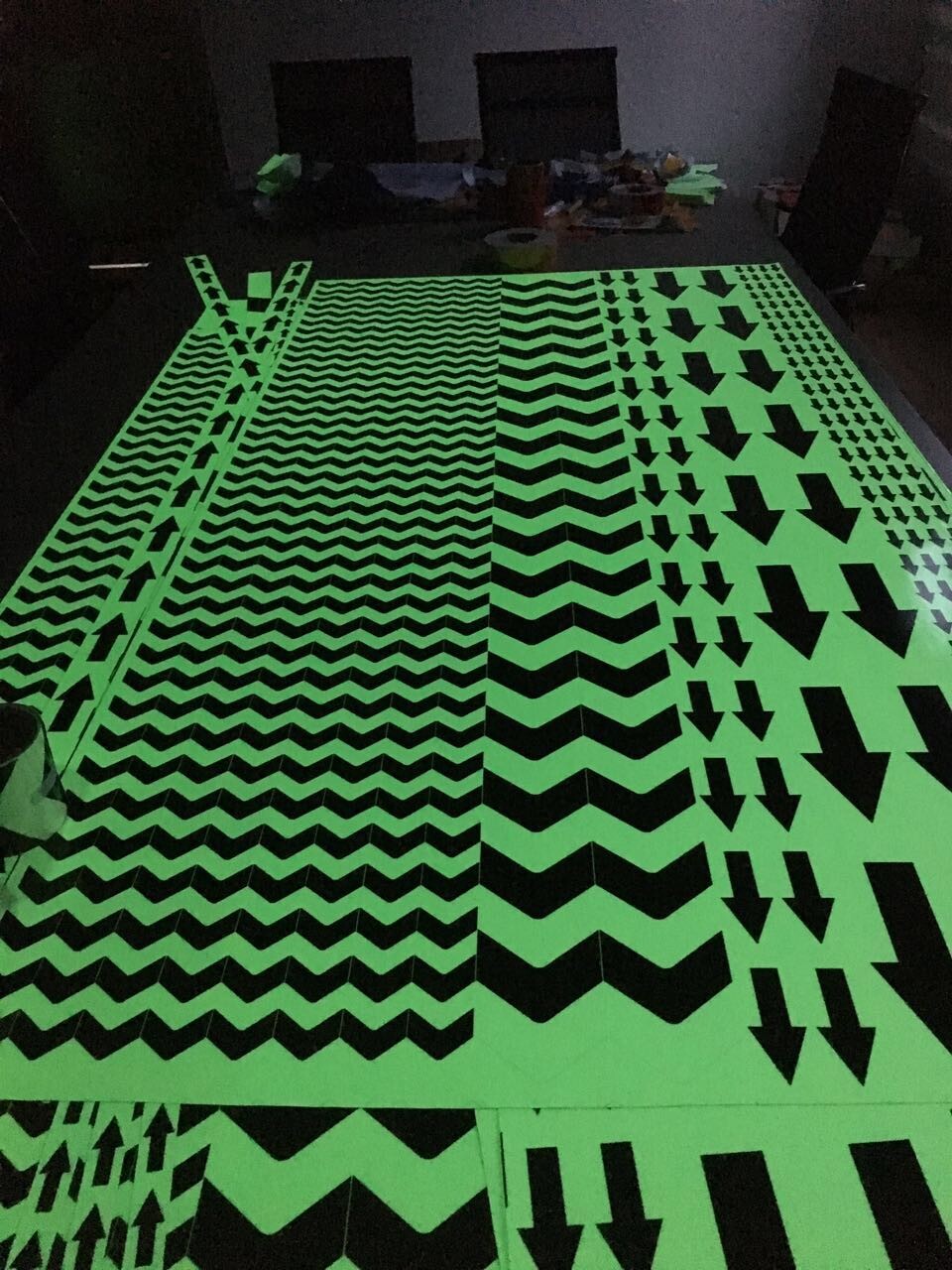
Glow Grip Tape,Luminous Adhesive Tape,Waterproof Glow In The Dark Tape,Glow In The Dark Reflective Tape
Kunshan Jieyudeng Intelligent Technology Co., Ltd. , https://www.jerrytape.com