On May 18th, the international academic journal Nature also published two major G-protein coupled receptor (GPCR) scientific research results, respectively, led by Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai University of Science and Technology, and Fudan University. The University College of Pharmacy completed it together.
The research team led by the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences successfully analyzed the three-dimensional structure of the human glucagon receptor (GCGR) full-length protein, revealing the regulation mechanism of the activation of different domains of the receptor protein; iHuman Institute of Shanghai University of Science and Technology The leading research team successfully analyzed the crystal structure of the seven-pass transmembrane region of human glucagon-like peptide 1 receptor (GLP-1R), revealing its allosteric regulation mechanism.
G protein-coupled receptor (GPCR) is the largest family of proteins in the human body and the largest family of drug target proteins. More than 40% of marketed drugs currently target GPCR. GPCR can be divided into four types: A, B, C and F. The B-type GPCR has 15 member receptors. Among them, the two major receptors that have made major breakthroughs are glucagon receptor (GCGR) and glucagon-like peptide-1 receptor (GLP-1R). They play an important role in regulating blood sugar levels in the human body.
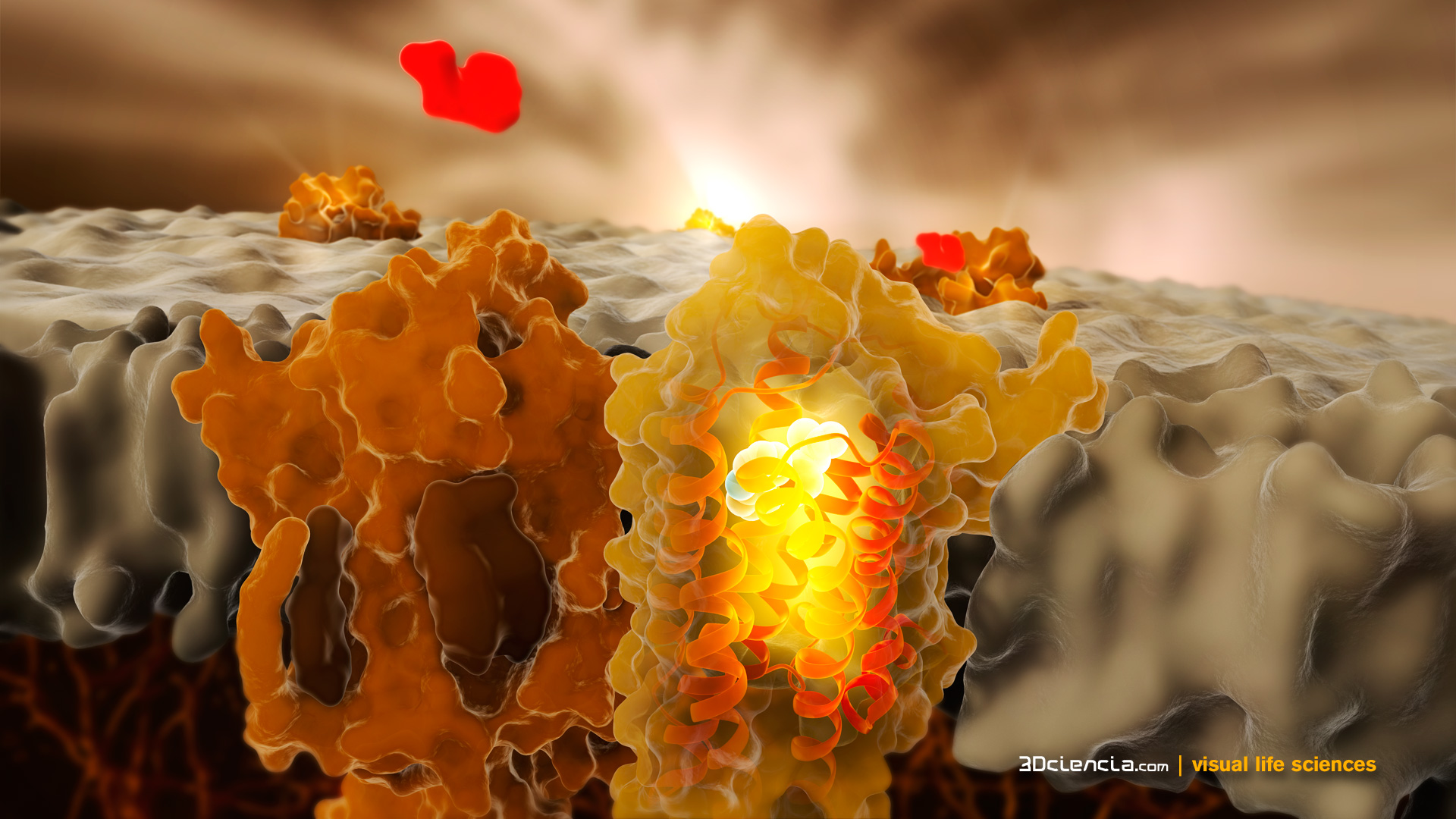
GCGR is involved in the regulation of blood glucose homeostasis and is an important target for the treatment of type 2 diabetes. The three research groups led by Shanghai Pharmaceutical Institute Wu Yuli, Wang Mingwei and Jiang Hualiang successfully interpret the full-length GCGR protein combined with a small molecule allosteric modulator (NNC0640) and an antagonist antibody through close cooperation with multiple disciplines. (mAb1) The crystal structure of the complex bound by the antigen fragment. It has been found that peptides linked to the extracellular and transmembrane domains of GCGR play a key role in the regulation of receptor activation through their conformational changes.
"Although it contains only 12 amino acids, this Stalk-linked peptide plays a vital role in acting as a 'switch' to regulate receptor function." Wu Xiaoli lamented the subtlety and efficiency of this magical switch.
GLP-1R is an internationally recognized therapeutic target for type 2 diabetes. A number of peptide drugs targeting this receptor have been marketed. However, small molecule oral drugs targeting GLP-1R have been used due to defects in peptide drugs that must be injected. It is a hot spot of concern for the international pharmaceutical industry. The iHuman Institute of Shanghai University of Science and Technology, the Shanghai Institute of Materia Medica of the Chinese Academy of Sciences and the School of Pharmacy of Fudan University have carried out joint research, breaking through the bottleneck of abnormal instability and low recombinant expression of GLP-1R in the natural state, and further stabilizing the receptor by means of small molecule antagonists. The protein, for the first time, obtained the crystal structure of the receptor seven times in the transmembrane region (non-activated state) with a resolution of 2.7 angstroms. At the same time, the scientists speculated on the possible activation of the G-type G-coupled receptor family of GLP-1R. These findings provide important clues for the development of small molecule oral drugs that target GLP-1R.
YT-M95
YT-M95
Shenzhen Sunshine Technology Co.,Ltd , https://www.shenzhenyatwin.com