Treatment of arthritis, Aberdeen's new drug has achieved 3 positive developments
June 06, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];AbbVie announced today that its Phase 3 clinical trial SELECT-EARLY has achieved positive top-line results. The results showed that in contrast to methotrexate, two doses of upadacitinib (15 mg and 30 mg once daily) reached ACR50 at 12 weeks (ACR20/50/70 is the American College of Rheumatology standard, indicating The primary endpoint of 20%/50%/70% improvement in joint swelling reached the primary endpoint of clinical remission at 24 weeks, as well as all secondary endpoints.

Rheumatoid arthritis affects approximately 23.7 million people worldwide and is a chronic and debilitating disease. In developed countries, the incidence rate of adults is between 0.5% and 1%, the highest incidence rate in middle age, and the incidence rate in women is 2.5 times that of men. In 2013, 38,000 people died of the disease. Although the cause of the disease is unclear, it may be affected by a combination of genetic and environmental factors, causing the body's immune system to attack the joints and cause disease. Methotrexate is a first-line treatment of commonly used rheumatoid arthritis, but many patients do not respond or are intolerant. Effective early intervention in rheumatoid arthritis is critical to controlling disease progression, preventing permanent joint damage and impaired physical function. Despite the availability of a variety of treatments, many patients with rheumatoid arthritis have not yet achieved the goal of clinical remission or low disease activity (LDA).
Upadacitinib, developed by Abbott, is a selective inhibitor of oral Janus kinase subtype 1 (JAK1). JAK belongs to the cytoplasmic tyrosine kinase family, whose function is to transduce cytokine (such as interferon)-mediated signaling. There are four JAK subtypes with overlapping bindings between subtypes. Inhibitors of this family of kinases have been shown to treat certain inflammatory and autoimmune diseases such as rheumatoid arthritis and Crohn's disease. However, the first generation of drugs lacked subtype selectivity, resulting in dose-dependent side effects of the drug. Upadacitinib is a second generation Janus kinase inhibitor that is highly selective for the JAK1 subtype. Upadacitinib is conducting clinical studies in a variety of indications such as rheumatoid arthritis, psoriatic arthritis, Crohn's disease, ulcerative colitis, ankylosing spondylitis and atopic dermatitis.
SELECT-EARLY is a phase 3 multicenter, randomized, double-blind, parallel-group, active comparative study designed to evaluate the updacitinib compared with methotrexate in patients who have not received methotrexate. The safety and efficacy of drugs in the treatment of adult patients with moderate to severe rheumatoid arthritis. The primary endpoint included the proportion of patients achieving ACR50 at 12 weeks compared with methotrexate, and clinical remission assessed at 34 weeks with DAS28 [CRP] (disease activity score with C-reactive protein [CRP] values). . The key secondary endpoints included the proportion of patients achieving ACR20, ACR70, and LDA, and the secondary endpoints included changes in the modified total score (mTSS) and the Health Assessment Questionnaire Disability Index (HAQ-DI). The trial is still ongoing, including a 48-week randomized, double-blind treatment period followed by a long-term follow-up study of up to 4 years.
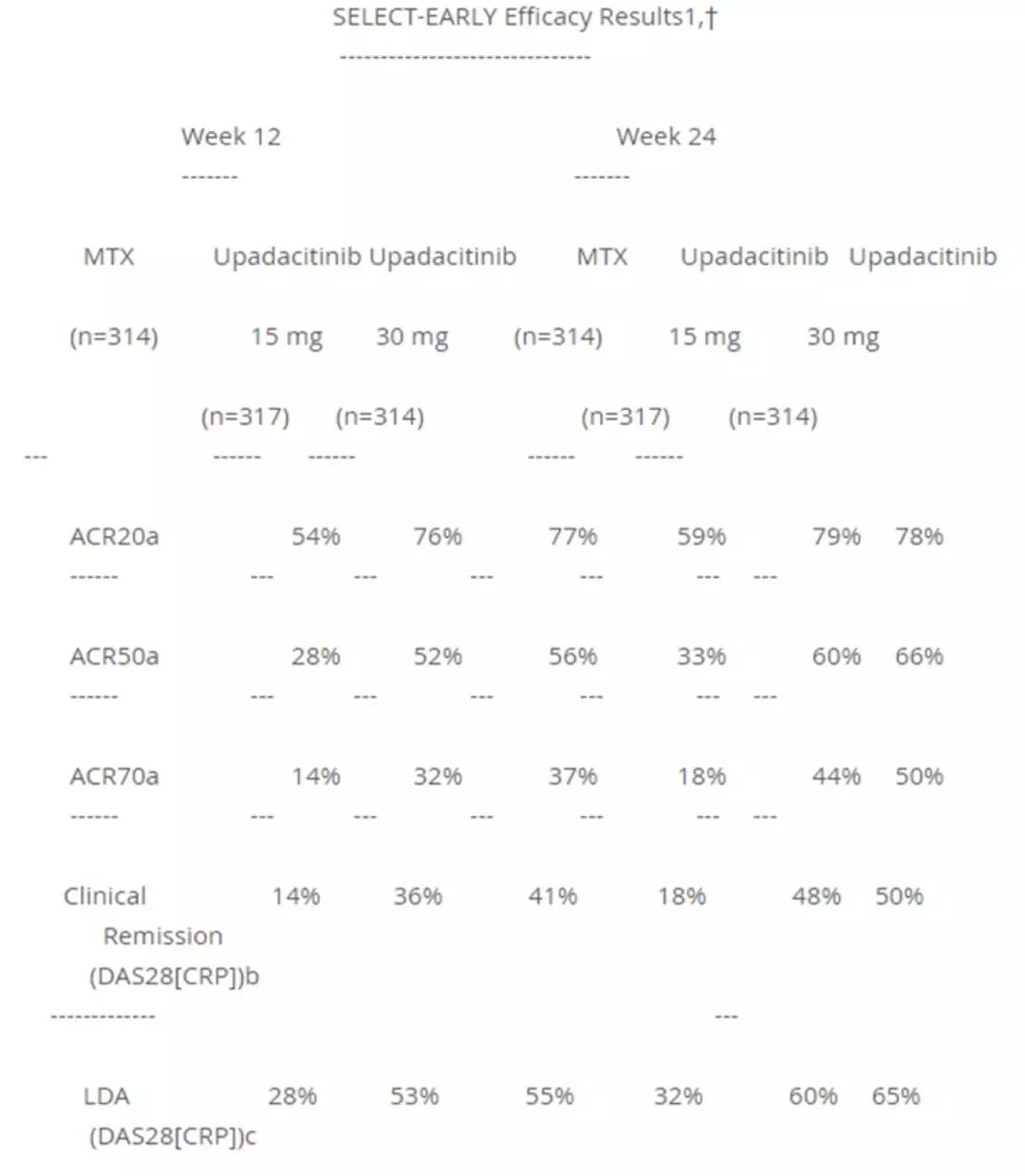
â–² SELECT-EARLY clinical trial results (Source: AbbVie official website)
The results of the trial showed that at the 12th week and the 24th week, both patients with higher doses of upadacitinib monotherapy had better relief at 12 weeks and 24 weeks. And at 24 weeks, upadacitinib significantly inhibited radiographic progression (mTSS assessment) compared to baseline, inhibiting joint damage in patients. Because joint damage can lead to permanent loss of function and disability, it is especially important for patients with rheumatoid arthritis. In addition, the safety of upadacitinib is consistent with previously reported results and no new safety signals have been identified.
Dr. Michael Severino, Aberdeen Development and Chief Scientific Officer and Executive Vice President of Research, said: "Aibowei will submit regulatory submissions for upadacitinib in the second half of 2018 to treat rheumatoid arthritis. SELECT-EARLY as the fifth key 3 Phase clinical trials provide a wealth of evidence that will further support upadacitinib as an important new treatment option for patients with rheumatoid arthritis."
Dr. Ronald van Vollenhoven, director of the Rheumatology Immunology Center in Amsterdam, and professor of rheumatology at the University of Amsterdam and Free University, said: "Upadacitinib's two different doses of monotherapy have reached the expected clinical level within half a year. The results of this goal are very encouraging. The results of SELECT-EARLY show that upadacitinib can be the most additional monotherapy, to control the progression of the disease in the early stages of rheumatoid arthritis, and has the potential to reduce the number of patients who have not received methotrexate. The risk of permanent bone damage and joint damage."
We look forward to Aberdeen's successful submission of its global regulatory submissions in the second half of the year to bring the gospel to rheumatoid arthritis patients as soon as possible!
Reference materials:
[1] Upadacitinib Monotherapy MeetsAll Primary and Ranked Secondary Endpoints Versus Methotrexate in a Phase 3 Study in Rheumatoid Arthritis
[2] AbbVie official website
[3] Courier | The effect is better than Humira, the new drug of arthritis reaches the end of the 3rd phase
Stirling Cooler,Twinbird Stirling Cooler,Cryogenic Stirling Cooler,Miniature Stirling Cooler
Shandong Freedoms Technology Co.,Ltd , https://www.sdfreedomtech.com