Summary of recent research progress in the field of cancer (05.28)
May 28, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];1. AstraZeneca lung cancer new drug significantly prolongs survival, reaching the end of phase 3
Recently, AstraZeneca and its global biologics research and development department, MedImmune, announced the positive results of Phase 3 clinical trial PACIFIC. The study evaluated the efficacy of Imfinzi (durvalumab) in patients with unresectable third-stage non-small cell lung cancer (NSCLC) who did not progress after receiving platinum-based chemotherapy and radiation therapy.

Lung cancer is one of the most serious cancer deaths in the world. NSCLC is the most common type of lung cancer, of which three-stage NSCLC accounts for about one-third. Most patients with stage III NSCLC are diagnosed as unresectable. The standard treatment regimen for these patients is chemotherapy and radiotherapy, followed by active monitoring. The prognosis is not optimistic.
Imfinzi (durvalumab) is a human monoclonal antibody that binds to PD-L1 and blocks the interaction of PD-L1 with PD-1 and CD80, counteracts tumor immune evasion strategies, and releases inhibition of immune responses.
PACIFIC is a randomized, double-blind, placebo-controlled, multicenter, phase 3 clinical trial evaluating the efficacy of Imfinzi in patients with unresectable third-stage NSCLC who have not progressed after receiving platinum-based chemotherapy and radiation therapy. . A total of 713 patients were enrolled in the study. The primary endpoints were progression-free survival (PFS) and overall survival (OS). Secondary endpoints included landmark PFS and OS, overall response rates, and duration of remission.
The data released this time is a mid-term analysis of the plan conducted by the Independent Data Monitoring Committee. The results showed a significant statistically and clinically significant improvement in OS in patients receiving Imfinzi compared to placebo, reaching the second of the two primary endpoints of the study. Data from the first primary endpoint, PFS, was released in May last year, demonstrating a median improvement of 11.2 months. In addition, the safety and tolerability characteristics of Imfinzi are consistent with the PFS analysis report.
2. Say goodbye to chemotherapy! Aberdeen's new leukemia drug arrives at the end of phase 3
AbbVie announced that its Phase 3 clinical trial, iLLUMINATE (PCYC-1130), reached the primary endpoint of progression-free survival (PFS) improvement. This study evaluated the efficacy of Imbruvica (ibrutinib) in combination with Gazyva (obinutuzumab) in the treatment of patients with chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL).

CLL is the most common form of leukemia in adults. It is a cancer that develops from cells in the bone marrow that later mature into certain white blood cells (lymphocytes). SLL is a slow-growing lymphoma that is biologically similar to CLL, where too many immature white blood cells cause lymph nodes to become larger. At present, the treatment of CLL/SLL is still limited to chemotherapy. Once the disease recurs, the overall survival of such patients is usually only 2 to 3 years.
Imbruvica (ibrutinib), a collaboration between Abbott Pharmacyclics and Janssen, is the first of its kind, a daily oral therapy that works by blocking Bruton's tyrosine kinase (BTK). BTK is a key signaling molecule in the B cell receptor signaling complex that plays an important role in the survival and spread of malignant B cells and other severely debilitating diseases. The WuXi PharmaTech Group Co., Ltd. has the privilege of assisting synthetic drugs to be marketed in China last year to treat patients with CLL/SLL/MCL who have received at least one treatment.

The current iLLUMINATE is a randomized, multicenter, open-label, phase 3 study evaluating the efficacy of chlorambucil and Gazyva combination therapy in patients with CLL/SLL initial treatment compared with Imbruvica and Gazyva. . In this study, patients were randomized to receive 6 consecutive cycles of Imbruvica 420 mg plus Gazyva 1000 mg, or chlorambucil plus Gazyva 1000 mg. The primary end point was the PFS assessed by the Independent Review Committee (IRC), and the secondary endpoints included overall response rate and minimum residual disease (MRD) negative response rate.
The results showed that patients treated with Imbruvica plus Gazyva had significant clinical and statistical differences in PFS compared with controls, reaching the primary end point of the study. Based on these data, Pharmacyclics and Janssen are working with regulators to advance the therapy approval process. If approved, it will be the first CD20 combination therapy in the first-line CLL treatment that does not include chemotherapy.
3. Metastatic prostate cancer has new drugs! Greatly improve absorption efficiency
Recently, Sun Pharmaceutical Industries and Churchill Pharmaceuticals jointly announced that the US FDA has approved the application for the innovative drug Yonsa. Yonsa is an innovative pharmaceutical formula for abiraterone acetate that will be used in combination with methylprednisolone for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC).

Prostate cancer is the second most common cancer among men in the United States. In 2017, about 160,000 men were diagnosed with prostate cancer, and nearly 27,000 people are expected to die from the disease. About 10-20% of prostate cancers are castration-resistant, and up to 90% of these patients develop bone metastases. The relative five-year survival rate of patients with metastases was 30%. Hormone therapy is one of the common treatments for prostate cancer that has metastasized. Because prostate cancer cells rely on androgen to help them proliferate, cutting off the supply of androgen can cause tumor cells to die or delay their growth.
Abiraterone acetate is a hormone therapy that inhibits androgen synthesis. Abiraterone acetate is converted into abiraterone in vivo, which is an inhibitor of CYP17 enzyme, which is expressed in testis, adrenal gland and prostate cancer tissues and is an essential protease for androgen biosynthesis.
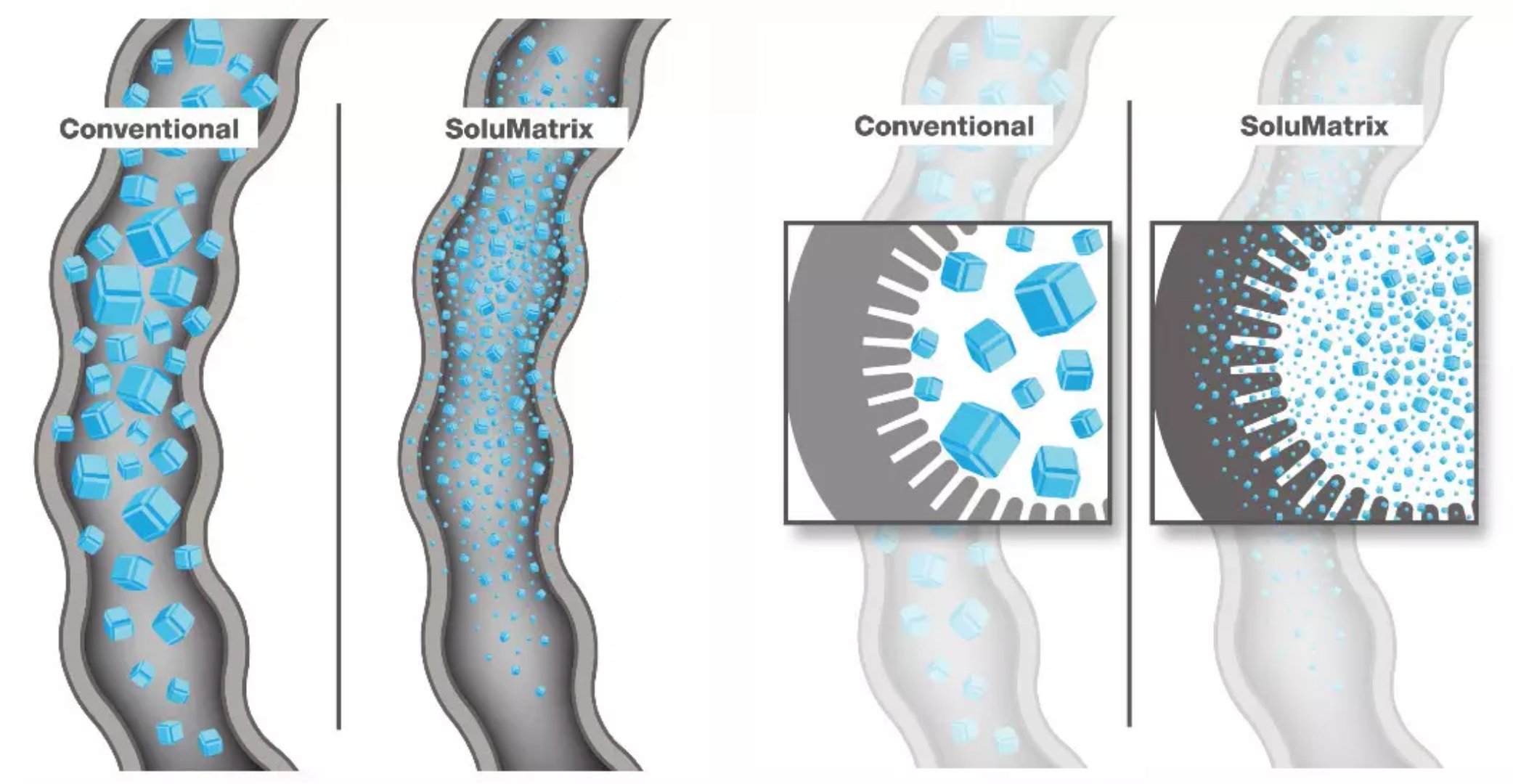
â–²Using SoluMatrix Fine Particle Technology technology can greatly increase the solubility and absorption efficiency of drugs
(Source: Churchill Pharmaceuticals official website)
Yonsa is unique in its use of Churchill's SoluMatrix Fine Particle Technology, an innovative pharmaceutical manufacturing technology. This technique grinds the drug particles into powders less than 1 micron in diameter while preventing the powder from re-aggregating. Drug particles produced using this technique are 10-200 times smaller than conventional drug particles, which greatly increases the solubility of oral drugs and the efficiency of absorption by the body.
In the closed open-label, randomized phase 2 clinical trial called STARR, a combination of 500 mg of Yonsa and methylprednisolone daily with 1000 mg of Zytiga (traditional formulation of abiraterone acetate) and prednisone (prednisone) Compared to combination therapy, therapeutic equivalency is achieved in reducing androgen levels in the patient's serum.
4. First-line treatment of lung cancer, Keytruda combination therapy significantly extends OS and PFS !
Recently, WuXi PharmaTech partner Merck has announced an important announcement: its heavy immunotherapy Keytruda combined with chemotherapy as a first-line therapy for non-small cell lung cancer, in a phase 3 clinical trial, while achieving a total survival The primary study endpoint for (OS) and progression-free survival (PFS). These results are expected to give Keytruda a breakthrough in regulation.

Lung cancer is one of the most deadly cancers. Non-small cell lung cancer (NSCLC) is the most common type, accounting for approximately 85% of all lung cancer cases. In the United States, the 5-year survival rate of lung cancer after diagnosis is only 18%, and the treatment prospects are not optimistic. In recent years, the rise of tumor immunotherapy has brought about major changes in the treatment of lung cancer. Keytruda and other immunotherapy against PD-1/PD-L1 pathway has also made a breakthrough in the treatment of lung cancer. In 2016, Keytruda was approved as a first-line therapy for patients with non-small cell lung cancer with high PD-L1 expression.
A phase 3 clinical trial called KEYNOTE-407 evaluated the potential of combination therapy with Keytruda and carboplatin-paclitaxel or albumin-binding paclitaxel (nab-paclitaxel) in the treatment of lung cancer. The trial enrolled 560 patients with metastatic squamous non-small cell lung cancer who did not receive any systemic therapy. Subsequently, these patients received a combination of Keytruda+ chemotherapy or chemotherapy alone.

After evaluation by the Independent Data Surveillance Commission (DMC), Merck announced the interim analysis of KEYNOTE-407, which showed that the combination of Keytruda and chemotherapy significantly prolonged overall survival and progression-free survival. The two main study endpoints. Specific data for the study will be announced at the ASCO Annual Meeting to be held shortly. Merck has submitted a supplemental biologics license application (sBLA) for Keytruda combination therapy to the US FDA for this indication. Keytruda is currently evaluating its potential to treat a variety of cancers in approximately 750 clinical trials.
5. A new breakthrough in 30 years? Three new positive treatments for severe bladder cancer
Recently, Sesen Bio, a company developing new antibody drug conjugate (ADC) therapy, announced that an ongoing phase 3 clinical trial of VISTA has a positive 3-month interim data. In this study, the researchers used a new ADC therapy, Vicinium, to treat patients with high-grade non-muscle invasive bladder cancer (NMIBC).

Bladder cancer is the sixth most common cancer in the United States, and approximately 80% of patients are NMIBC. In such diseases, cancer cells are located in the bladder or have grown into the bladder lumen, but have not yet spread to muscle or other tissues. Patients who underwent initial surgical resection have a high recurrence rate, and more than 60% will receive BCG immunotherapy. Although BCG is effective in many patients, tolerance problems have been observed and many patients experience disease recurrence. If the BCG is not effective or the patient needs to receive BCG for a long time, the recommended treatment is to completely remove the bladder. In the past 30 years, the medical community has had little innovation in treating this type of cancer.
Vicinium may offer patients a new treatment option. It consists of a recombinant fusion protein that targets the epithelial cell adhesion molecule (EpCAM) antigen on the surface of tumor cells to provide an effective protein load - Pseudomonas exotoxin A (ETA). EpCAM has been shown to be overexpressed in NMIBC cells, but rarely or even not on normal bladder cells. Vicinium is linked by a stable genetically engineered peptide, ensuring that the payload remains attached until it is internalized by cancer cells, reducing the risk of toxicity to healthy tissue and increasing safety.
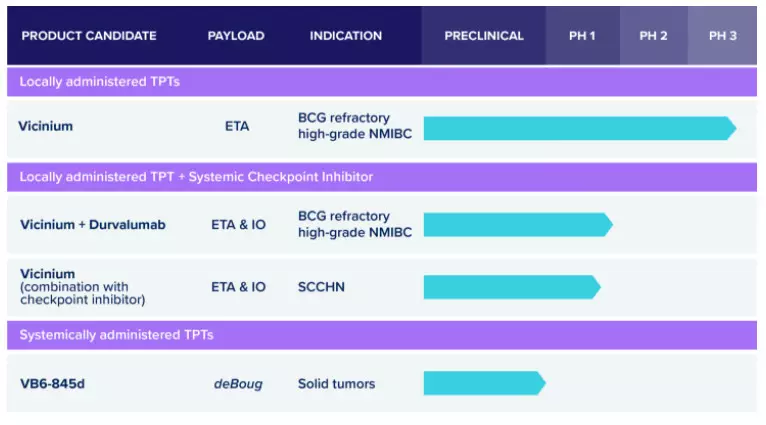
â–²Sesen Bio's R&D product line (Source: Sesen Bio official website)
VISTA is an open-label, multicenter, one-arm, phase 3 clinical trial evaluating the efficacy and tolerability of Vicinium in high-grade NMIBC carcinoma in situ (CIS) or papillary carcinoma in 129 patients. These patients had previously received BCG treatment and the primary endpoint of the study was the patient's complete response rate (CR). The 3-month mid-term results showed that among the CIS patients in cohort 1, 72 patients with evaluable data had a CR of 39%. In the 5 evaluable patients in cohort 2, CR was 80%. The CR for all 77 CIS patients treated for three months was 42%. The 3-month efficacy of cohort 3 patients with papillary carcinoma showed that 34 patients who were evaluable showed a 68% recurrence-free rate. Initial safety results showed that Vicinium was well tolerated in the VISTA trial, with 72% of patients experiencing grade 1 or 2 adverse events.
Reference materials:
[1] AstraZeneca caps its big win in lung cancer with positive survival data for Imfinzi — and what a relief it is
[2] IMBRUVICA® (ibrutinib) Plus GAZYVA® (obinutuzumab) Phase 3 iLLUMINATE Trial for First-Line Therapy of Chronic Lymphocytic Leukemia (CLL) Patients Met Primary Endpoint
[3] Sun Pharma Announces USFDA Approval of YONSA® (abiraterone acetate) To Treat Metastatic Castration-Resistant Prostate Cancer In Combination With Methylprednisolone
[4] Merck crushes Keynote-407 study in frontline lung cancer, hitting co-primaries and setting up another quick OK
[5] Sesen's PhIII bladder cancer data send stock south as investors fret over safety
Original Title: Summary of Recent Research Progress in the Field of Cancer (No. 67)
Sodium Gluconate,High Quality Sodium Gluconate,Sodium Gluconate Details
SHANDONG BAISHENG BIOTECHNOLOGY CO., LTD , https://www.baishengbioproducts.com