Recently, Claret Medical announced that the FDA has approved the company's Sentinel Brain Protection System (CPS) for sale in the United States according to the reclassification criteria. Sentinel is the first and only device available in the United States to provide protection against stroke risk by intercepting and removing debris that has fallen off during transcatheter aortic valve replacement (TAVR) before it reaches the brain.
Sentinel
As more and more heart valve repair procedures are converted from open surgery to transcatheter aortic valve repair (TAVR) surgery, TAVR surgery is a less damaging option for many physically weak patients. However, TAVR also faces certain risks during the operation. During the TAVR operation, the embolic fragments will be released. These fragments will pose serious risks to many tissues and organs, especially the brain. These fragments will block the blood vessels in the blood. If they are produced in the brain, it will cause Stroke.
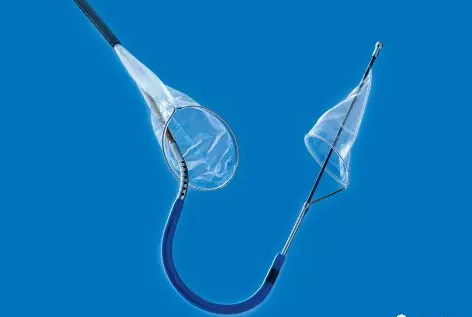
To address the shortcomings of TAVR surgery, Claret Medical developed the Sentinel Brain Protection System to optimize TAVR surgery.
Sentinel composition
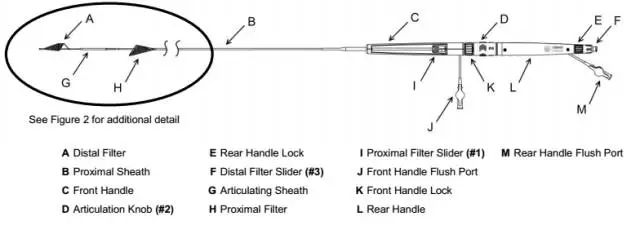
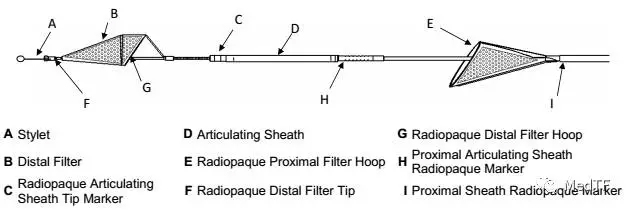
Sentinel use
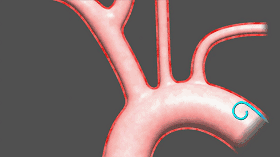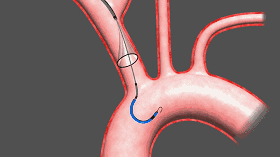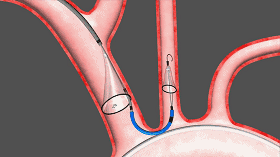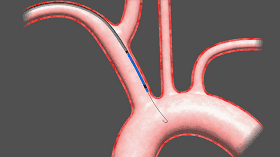
Sentinel includes two filters that are delivered to the target location in a very wonderful way, one of which is the brachiocephalic artery and the other is the left common carotid artery. The device was inserted from the right arm right iliac artery and placed at the border of the brachiocephalic artery and the left common carotid artery. Dangerous debris will be trapped by the filter of the device, after which the captured debris will be removed from the right arm along with the device.
Blowing Mouth,Disposable Blowing Mouth,Filter Spirometer Mouthpiece,Disposable Spirometer Mouthpiece
Hengshui Qifei Paper Products Co. LTD , https://www.hengshuiqifei.com