WHITE PAPER: Virotherapy Process Optimization
The use of a range of methods for the development of viral engineering for disease treatment has become a novel application, broadly defined as viral therapy, including viral vectors for gene therapy (Figure 1) and viruses with oncolytic function ( Figure 2). Although most of these products are in clinical development, the diversity of drug targets and the wealth of future opportunities are encouraging. Viral therapy is a virus-based technology. An important challenge is to clearly understand the quality and performance of the sample at any point in time, including early development, product production, and product packaging. The most important One point is the quantification of the virus. In the past, the quantification of viruses relied on methods such as plaque and electron microscopy. These methods required a large amount of labor, waited for a long time to obtain results, and the results obtained were highly subjective. However, viral drugs and vaccines based on the diversity of new virological technologies require new analytical methods. In this article, we will discuss how the virus counter 3100 real-time virus concentration monitoring function can significantly promote the development of viral therapy products.
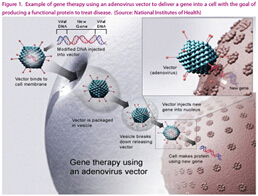
Reprogramming Viruses to Treat Disease
Gene therapy has been around for more than a decade, but it has been frustrated by the death of the patient. Fortunately, in 2006, gene therapy was clinically successful, so gene therapy is also known as a hot topic. More than 2,000 clinical trials have been conducted or completed in many different therapeutic areas and landmarks. The biggest difference between gene therapy and other viral therapies is that gene therapy delivers a healthy gene from the body to the patient's cells instead of a non-functional fragment. Depending on this method, this healthy gene may or may not continue to integrate into the host cell genome. In contrast, viruses of oncolytic cells can use a variety of methods to completely destroy tumor cells, rather than altering the genetic makeup of infected cells. Oncolytic cell virus therapy involves lysis, stimulating an anti-tumor immune response, encoding an enzyme that activates the prodrug to become an effective cytotoxin, inhibiting angiogenesis and radiation therapy sensitization.
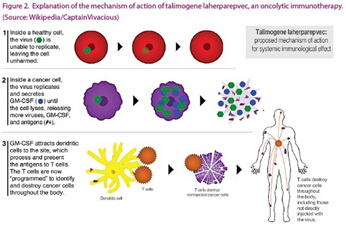
The principle and mechanism of oncolytic cell therapy is shown in Figure 2. This picture is created by Amgen. T-VEC is currently used to study the treatment of melanoma and other advanced cancers, using selective herpesvirus to selectively infect cancer cells. The secretory cell cytokine stimulates the immune response and has shown significant results in phase III clinical trials. In addition to cancer, viral therapy is also recommended for the treatment of other diseases, such as the use of bacteriophage to treat bacterial infections.
To date, many viruses have been evaluated in viral therapy applications (see Table 1).

These viruses were chosen because they target only specific cell types or are suitable for genetic manipulation. In order to develop these viruses into gene delivery vectors or therapeutic factors, these viruses must be scaled up and culture conditions optimized during the culture to obtain maximum yield. In the whole process, such as inoculation, volume expansion culture, sample collection, purification, concentration, virus packaging, etc., there are opportunities for delays and interruptions. Because many viral quantification methods take days or even weeks to interrupt the entire process, virus quantification is a big bottleneck. The Virus Counter 3100 (Figure 3) provides accurate, repeatable viral particle quantification results in minutes, allowing real-time monitoring of virus concentrations. The 96-well plate auto-loading system, designed for GMP requirements, is well suited to meet the requirements of large-scale virus production environments.
Figure 3. Virus Counter 3100 (top) and Autosampler (bottom)

Proof of Principle
Virus counters have been widely used for the counting of a variety of viruses, including gene delivery vectors and oncolytic functions. Here we present some test data to confirm the practicality of the virus counter rapid viral quantification in the field of viral therapy.
The accuracy of the virus counter is measured by detecting the slope and linear relationship of the serially diluted virus sample data. The closer this value is to 1, the more reliable the data generated by this machine is, the more it reflects the authenticity of the virus concentration.
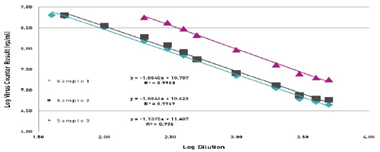
Figure 4 confirms this experiment, in this case the varicella virus was detected. Three different samples were tested for 11 dilutions with slopes of -1.0845, -1.0846, -1.14, and R2 of 0.995, 0.997 and 0.996, respectively, showing good accuracy.
Figure 4. Quantification of serial dilutions of Vaccinia virus usingthe Virus Counter.
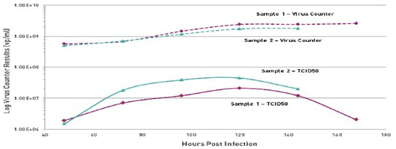
In the next example, a client wants to track the concentration of measles virus in HeLa cell culture. They used a virus counter to measure total viral particles and measured the infectivity of the virus with TCID50 (this method took 5-7 days) and compared the relationship between the two (Figure 5). Similar to most viruses, the number of infectious measles viruses is only a small fraction of the total viral population, in this case only 0.6% to 1.7%, suggesting that most of the virus cultured in this batch is non-infectious. Virus. Interestingly, after the virus was infected, the number of infectious viruses decreased significantly after the cells were cultured for more than 120 hours, while the total amount of the virus remained stable, suggesting that the effectiveness of the virus infection was adversely affected by the prolonged culture time.
Figure 5. Comparison of total particle count (Virus Counter) and infectivity (TCID50) of Measels virus grown in HeLa cells.
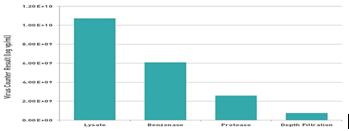
In addition, the use of a virus counter to track the concentration of viral particles in each step of the measles virus purification (Figure 6) demonstrates the importance of real-time quantification of the virus in virus purification optimization to avoid errors. Without real-time monitoring of virus counters, anyone who is consuming a virus therapy agent is groping in the dark, risking millions of dollars and the health risks of patients who need safe and effective products for treatment.
Figure 6. Tracking virus particle counts during harvest and purification.
About ViroCyt
ViroCyt, LLC was created with the goal of bringing game-changingsolutions to the Life Science industry. Our primary focus is replacing outdated technologies that slow down the pace of virus-related research and product development. Additional information can be found at http:// .com.
Anti Bird Netting,Anti Bird Net For Balcony,Anti Pigeon Net,Anti Bird Mesh
Changzhou Satidi Import and Export Co., Ltd. , https://www.guanjiejts.com