Medical Network August 16th, August 15th, CDE updated the review data, Qilu Pharmaceutical's recombinant anti-VEGF humanized monoclonal antibody injection (bevacizumab) new drug category 2 listing application has been accepted by CDE, the first A bevacizumab biosimilar drug officially took the footsteps of the market. The industry generally believes that the rapid listing of domestic monoclonal antibody drugs will further affect the market segment of the multinational pharmaceutical companies.
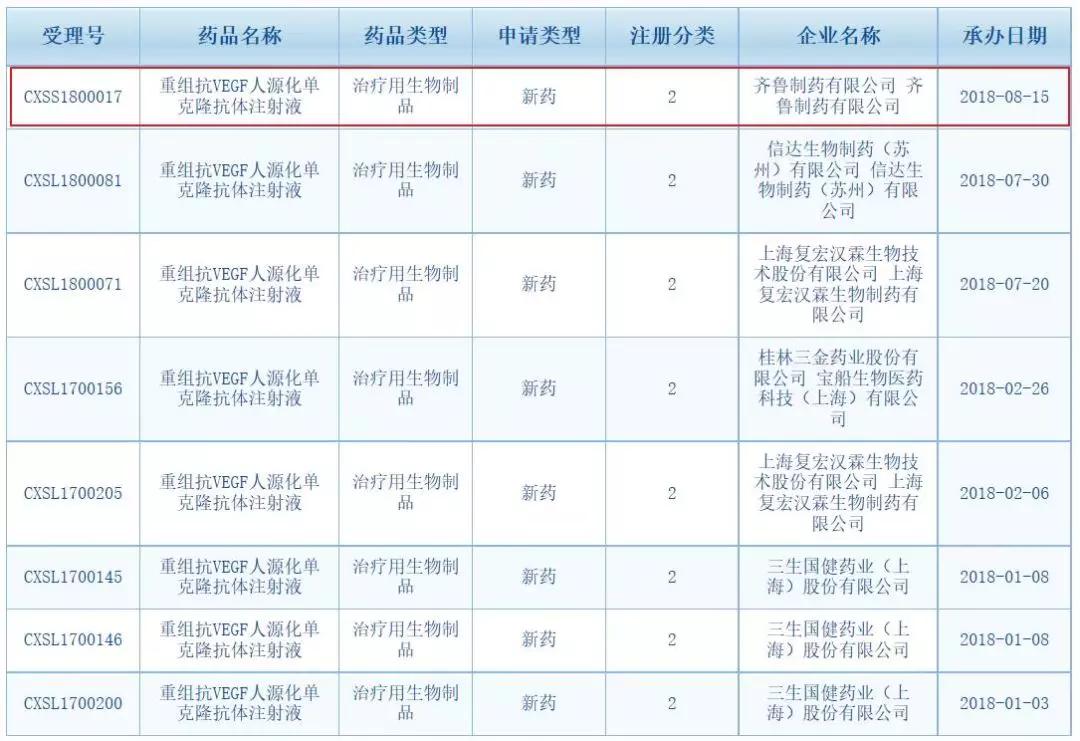

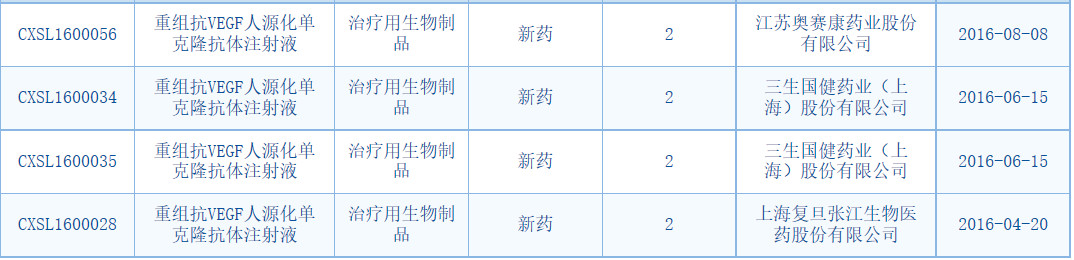
Domestic similar drugs racing
On the R&D side, the 2007 version of the “Regulations on the Administration of Drug Registration†requires “biological products to be declared in accordance with the procedures for new drug applicationsâ€; subsequently, in February 2015, the “Technical Guidelines for the Development and Evaluation of Biosimilar Drugs (Trial)†was issued, and the regulatory authorities The definition of the drug and the development path are more clear; in July 2016, the Drug Registration Management Measures (Revised Draft) regulates the concept of biosimilar drugs, and in the first seventy-seven articles, “the drug approval should focus on: biosimilar drugs and original research. The quality and efficacy of the drug are similar."
The monoclonal antibodies developed by domestic companies are concentrated in rituximab, trastuzumab, bevacizumab and other varieties, most of which follow the old regulatory path for new drug declarations. It can be seen from the CDE data that in addition to Qilu Pharmaceutical, Fuxing Subsidiary Shanghai Fuhong Hanlin, Xinda Bio, Sansheng Guojian, Baiaotai, Jiangsu Osei Kang, Guilin Sanjin and other enterprises have related varieties. R&D layout.
It is also known that in the first half of this year, a number of companies have started clinical trials of bevacizumab: Fosun adopts a differentiated strategy for phase III clinical trials of colon cancer indications, and HLX04 of Fuhong Hanlin was completed in early 2018. Phase I clinical trial, and phase III clinical trial of metastatic colon cancer in China in April this year; in addition, Cinda IBI305, Qilu QL1101 phase III clinical research on non-small cell lung cancer is progressing well.
The biosimilar drug market has the characteristics of “large space, high barriers and low price reductionâ€. Qilu Pharmaceutical has taken the lead in completing clinical trials and promoting the preliminary listing of bevacizumab, which means that a new round of domestic high-quality antibody drugs is on the market. Good, unmet clinical contradictions will be expected to be alleviated.
High level of competition is coming
On the market side, the biosimilar drug-targeted Roche's original research drug Avastin (bevacizumab), a humanized monoclonal antibody against VEGF, can be used alone or in combination with other chemotherapy regimens for colon cancer, breast cancer, and non- Treatment of advanced cancers such as small cell lung cancer, renal cell carcinoma, and glioblastoma multiforme. In 2017, Avastin's global sales exceeded 6.6 billion Swiss francs, ranking seventh in the global pharmaceutical sales rankings.
On April 14, 2017, the Ministry of Human Resources and Social Security issued the “Notice on the Determination of the Scope of Negotiation of the National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List in the Office of the Ministry of Human Resources and Social Security†to determine the list of 44 negotiable drugs. It contains 7 monoclonal antibody drugs, showing that the relevant departments of medical insurance fully recognize the clinical value of monoclonal antibodies. Roche's anti-tumor monoclonal antibody, the Three Musketeers, trastuzumab, rituximab and bevacizumab entered the negotiating table.
Domestic sales of bevacizumab have grown steadily and the market space is large. According to third-party data, the sales of bevacizumab in China are growing rapidly. In 2017, the sales of bevacizumab in domestic sample hospitals exceeded 500 million yuan.
Bevacizumab was negotiated in 2017 to include the use of colon cancer indications and non-small cell lung cancer indications in medical insurance, with price cuts approaching 60%. It is widely expected in the industry that medical insurance will continue to increase sales and drive sales. Quickly break through the sales of 1 billion yuan. If the market is fully extended in the field of multiple indications in the future, the market share of more than 5 billion yuan in the long-term will be divided by the first-time enterprises .
At the same time, with the zero-tariff anti-cancer drug and the special procurement of medical insurance, the price of bevacizumab in the local area is also facing increasing pressure on price reduction. Nowadays, in the face of the upcoming domestic biosimilar drugs, the situation of short-term and long-term incremental competition in the regional market will inevitably increase, and the high-level competition between Chinese pharmaceutical companies and multinational pharmaceutical companies has just begun.
Tetramisole Hcl,Albendazole Bolus,Multivitamin Premix,Diminazene Diaceturate
NINGBO VOICE BIOCHEMIC CO. LTD , https://www.medicine-voice.com