Section 6 PD-1/PD-L1 Tumor Immunotherapy will debut from Sanofi/Regeneration
April 08, 2018 Source: Sina Pharmaceutical
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Sanofi and its partner Regeneration Yuan recently announced that the European Medicines Agency (EMA) has accepted a marketing approval application (MAA) for the monoclonal antibody cemiplimab (REGN2810). This application seeks to approve the use of cemiplimab for the treatment of patients with metastatic squamous cell carcinoma (CSCC) and for the treatment of patients with locally advanced CSCC who are not suitable for surgical resection. In terms of US regulation, the FDA is also reviewing cemiplimab for the same indication.

The CSCC is one of the most common malignancies in the world, and the number of newly diagnosed cases is expected to increase each year. Although CSCC has a good prognosis in early detection, it becomes particularly difficult to treat if it progresses to the advanced stage, and the quality of life of patients is significantly reduced due to the disease. Advanced CSCC is the most deadly non-melanoma skin cancer. Currently, there are no drugs approved for the treatment of advanced CSCC.
The MAA submission was based on data from a critical, one-arm, open-label phase II clinical study, EMPOWER-CSCC 1, and two advanced CSCC extension cohorts in a phase I clinical study. Patients enrolled in these studies were patients with metastatic CSCC and patients with locally advanced CSCC who were not eligible for surgical resection:
(1) Data from the EMPOWER-CSCC 1 study was published in December 2017. The data showed that the overall response rate of cemiplimab monotherapy evaluated by the Independent Review Committee was 46.3%. At the time of data analysis, all patients were followed for at least For 6 months, the duration of median remission had not been reached, and 32 of 38 patients continued to remission. In terms of safety, the drug is basically identical to other anti-PD-1 formulations already on the market.
(2) Data from the Phase I expansion queue was also announced at the ASCO Annual Meeting in June 2017. The data showed that the total response rate of cemiplimab monotherapy was 46.2% (n=12/26), of which 2 were complete remission, 9 were partial remission, and 1 was not yet confirmed. Monotherapy The control rate was 69.2% (n=18/26). At the time of data analysis, the median follow-up time was 6.9 months (range: 1.1–13.8 months), and the median progression-free survival and overall survival were not met. In terms of safety, the most common treatment-related adverse events were fatigue (23.1%), and grade 3 and above adverse events included joint pain (3.8%), maculopapular rash (3.8%), fatigue (3.8%), aspartate aminotransferase AST increased (3.8%) and alanine aminotransferase ALT increased (3.8%).
Currently, these two studies are still in progress and the updated data will be announced at the medical conference held in 2018.
In September last year, based on the above-mentioned Phase I clinical study expansion cohort data, the FDA has awarded cemiplimab a breakthrough drug qualification (BTD) for the treatment of advanced CSCC. The above phase II clinical study data is also the key data for Sanofi and Regeneration Yuan to submit the drug to the FDA for the treatment of advanced CSCC biologics (BLA). At the end of last year, both parties have initiated the submission of BLA to the FDA. Rolling submission means that you can submit some of the completed content in the BLA first, without having to wait for all the parts to complete before submitting.
Last month, Renaissance CEO Leonard Schleifer said the BLA rollover is expected to be completed in the first quarter of 2018 and is expected to be approved by the FDA by the end of 2018. Currently, Sanofi and Regeneration are actively preparing for the cemiplimab listing.
Launched paragraph 6 PD-1/PD-L1 tumor immunotherapy
Cemiplimab is an experimental, fully humanized monoclonal antibody that targets the immunological checkpoint PD-1 (programmed cell death protein-1), which was created and optimized by Regen's proprietary Velocimmune technology platform. It is developing globally with Sanofi. In addition to the indications for CSCC, the two sides are also developing other indications for the drug, including basal cell carcinoma and first- and second-line treatment of non-small cell lung cancer.
Sanofi is quite confident in the regulatory approval of cemiplimab for the treatment of CSCC indications. The company previously said that cemiplimab achieved a 46% overall response rate in Phase II clinical trials and is the highest data observed to date for any of the PD-1 targeted agents (including those already marketed) for the treatment of solid tumors. The industry is also expected to have a high level of drug, if approved, the drug will become the first targeted drug to treat CSCC, and will become the sixth PD-1/PD-L1 tumor immunotherapy on the market.
At present, there are five PD-1/PD-L1 tumor immunotherapy on the market, namely Keytruda (pembrolizumab, target PD-1) of Merck, Opdivo (nivolumab, target PD-1) of Bristol-Myers Squibb, Rocent's Tecentriq (atezolizumab, target PD-L1), AstraZeneca's Imfinzi (durvalumab, target PD-L1), Pfizer/Merck's Bavencio (avelumab, target PD-L1). In addition, Novartis PDR001 (target PD-1), Incyte/Jiangsu Hengrui INCSHR-1210 (target PD-1), and Baekje Shenzhou BGB-A317 (target PD-1) are also in stage III clinical trials. Development, another large wave of PD-1/PD-L1 immunotherapy is still in the early stage I / II development. (See: China PD-1/L1 market status: 17 pharmaceutical companies layout 22 drugs)
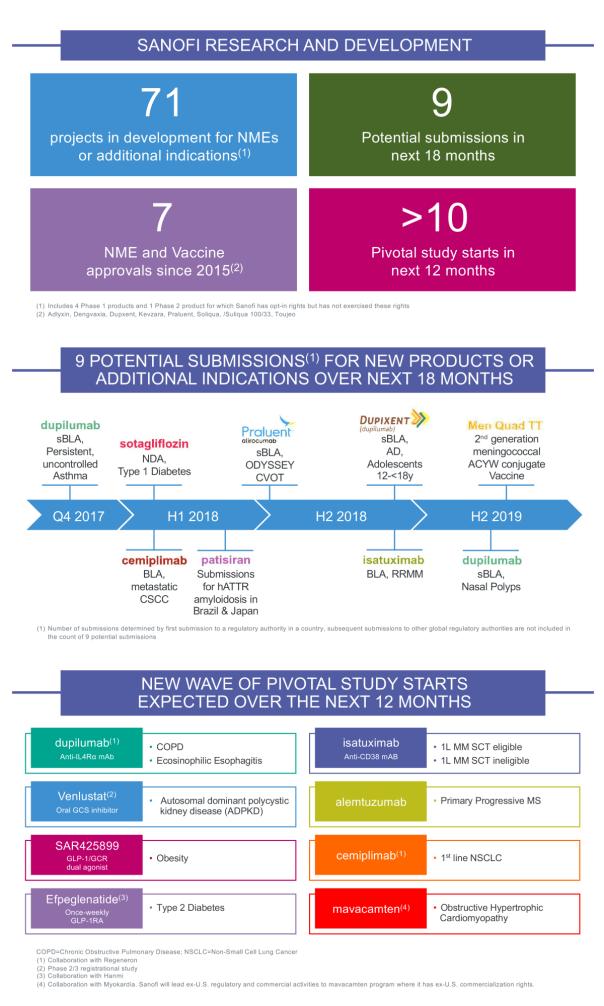
It is worth mentioning that cemiplimab is also one of the key development drugs in the 2020 Strategic Blueprint released by Sanofi at the end of last year. According to the blueprint, Sanofi's R&D pipeline includes 71 R&D projects, including 37 new molecular entities (NMEs) and innovative vaccines; the company plans to launch a new round of at least 10 key Phase III clinical studies in 2018, Eight innovative drugs were evaluated; in addition, in the 18 months to June 2019, Sanofi plans to submit nine new drugs or additional indications, and cemiplimab is one of two anticancer drugs. (See: Sanofi released the "2020 Strategy Blueprint": three-pronged research and development strategy is full of points) (Sina Pharmaceutical Compilation / newborn)
Articles, pictures reference source: Sanofi's PD-1 inhibitor starts EU review for skin cancer
Laser Scanning Confocal Microscope
Laser Scanning Microscope,Laser Confocal Microscope,Scanning Confocal Microscope,Microscopia Laser Confocal
Ningbo ProWay Optics & Electronics Co., Ltd. , https://www.proway-microtech.com