Novartis releases long-term follow-up results of CAR-T therapy Kymirah
February 05, 2018 Source: WuXi PharmaTech
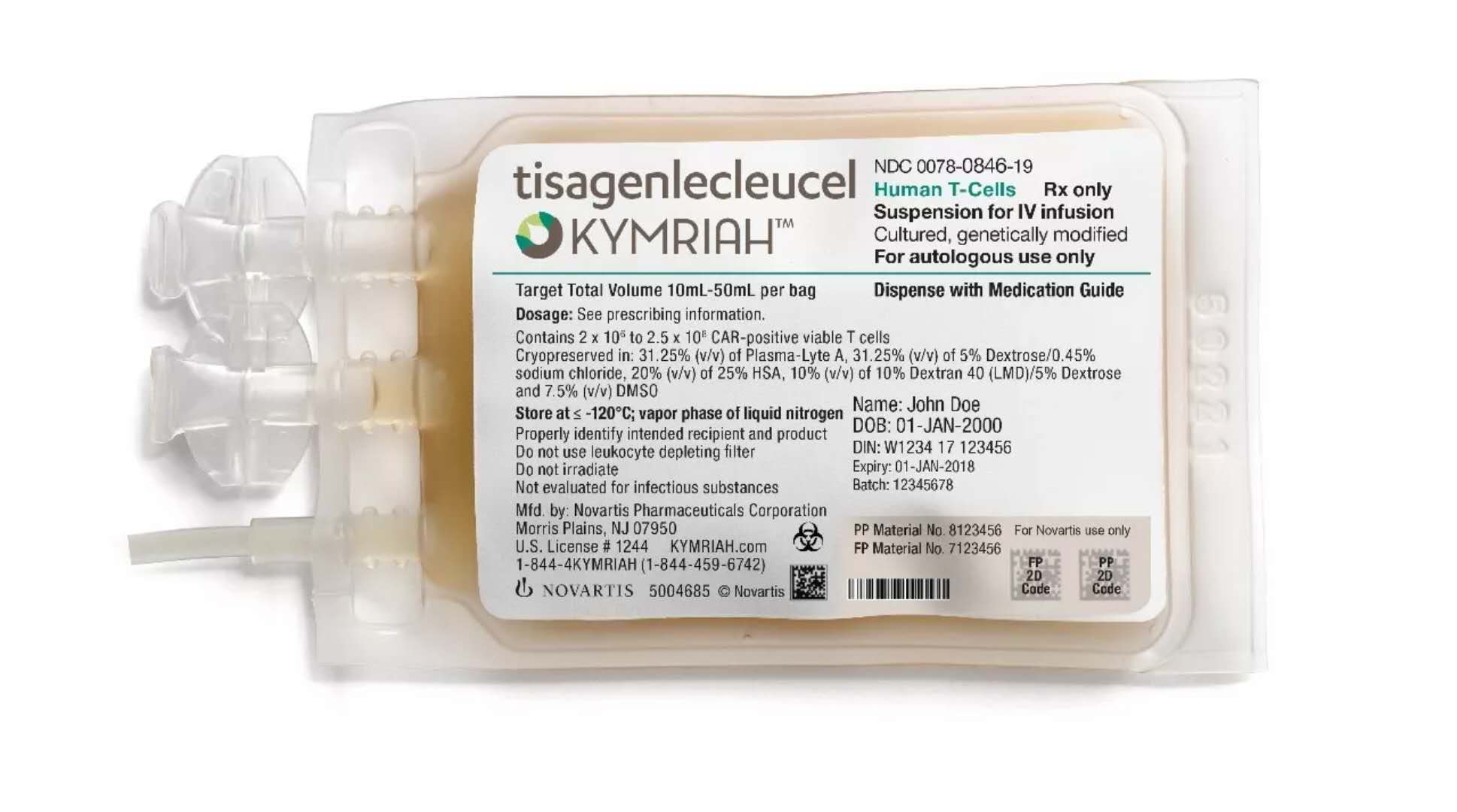
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Novartis has announced the results of the key clinical trial ELIANA. The project tracked the efficacy of CAR-T therapy KymriahTM (tisagenlecleucel, formerly known as CTL019) for children with relapsed or refractory B-cell acute lymphoblastic leukemia (r/r ALL) and young adults under 25 years of age. The latest issue of the New England Journal of Medicine (NEJM).

In August 2017, Kymriah became the first FDA-approved chimeric antigen receptor T cell therapy for patients with relapsed or refractory B cell acute lymphoblastic leukemia under 25 years of age. This approval is based on the early results of the ELIANA study conducted by the University of Pennsylvania and the Children's Hospital of Philadelphia (CHOP) to support the efficacy of Kymriah.
The ongoing study by ELIANA performed a follow-up analysis of 75 infusion patients for 3 months or longer to track the persistence and long-term safety of Kymriah. Among them, the overall response rate was 81% (95% CI: 71% - 89%) in patients with a median follow-up of more than one year. 60% of patients achieved complete remission (CR), 21% achieved CR, but blood cell count recovery was incomplete (CRi), and no minimal residual disease (MRD) was found in all patients (95% on day 28 [58/ 61]). The median follow-up time was 13.1 months.
In patients who achieved CR / CRi, the median duration of remission was not reached. Remission was long-lasting, with a recurrence-free survival rate of 80% at six months. 50% event-free survival rate of 73% at 6 months (95% CI: 60%-82%) and 12 months (95% CI: 35%-64%), did not reach median event-free survival . The overall survival of 75 infusion patients was 90% at 6 months (95% CI: 81%-95%) and 76% at 12 months (95% CI: 63%-86%).
Kymriah cells were detected in patients who received input for up to 20 months. The median duration of Kymriah was 168 days (continued range: 20-617 days; n = 60 patients with CR/CRi). All patients with remission showed B-cell dysplasia (low or missing B-cell count), which is a targeted effect of the expected Kymriah treatment.
95% of patients experienced different levels of treatment-related adverse events (AEs). The most common non-hematologic adverse events were cytokine release syndrome (CRS, 77%), fever (40%), and decreased appetite (39%). , fever neutropenia (36%) and headache (36%). Seventy-three percent of patients experienced grade 3/4 treatment-related AEs. CRS occurs in 77% of patients, a complication that is known to Kymriah as a cell that may be activated in a patient. To ensure patient safety, Kymriah is only available through a network of accredited treatment centers that are fully trained in Kymriah use and appropriate patient care.

â–² Dr. Shannon L. Maude of the Perelman School of Medicine at the University of Pennsylvania (Source: CHOP)
The main research author, Assistant Professor of Pediatrics at the Children's Hospital of Philadelphia, Dr. Shannon L. Maude of the Perelman School of Medicine at the University of Pennsylvania, said: "We are encouraged by the results confirmed by Kymriah in the patient population. These patients have limited treatment options. And it is now possible to achieve lasting remission, which translates into long-term survival. The long-term follow-up of the ELIANA study not only confirms that this is a potential change paradigm, but also provides increasing evidence of the key role of cell function. Continuous amplification and Kymriah present in the patient's system are associated with the durability of the clinical response."
Novy Executive Vice President, Dr. Samit Hirawat, Head of Global Drug Development, Oncology, said: "Kymriah is the first FDA-approved CAR-T cell therapy, showing the potential to be a clear treatment, relapsed or refractory Children and young people with sexual ALL provide early, deep and long-lasting relief. These data demonstrate our commitment to Novartis. We will continue to conduct CAR-T cell therapy research to enable as many patients as possible to receive this treatment."
We congratulate Novartis for the excellent results and expect more patients to benefit from this research.
Reference materials:
[1] Novartis official website
[2] Novartis Announces NEJM Publication of Updated Analysis from ELIANA Trial Showing Longer-Term Durable Remissions With Kymriah in Children, Young Adults With r/r ALL
Our polypropylene cap is combined by three parts which includes the outer-cap, inner cap and the medicinal synthetic polyisoprene gasket. The function of the outer cap can give us a force to open the cap .At the same time ,it also helps to prevent the external pollution. The polyisoprene gasket locates in the middle place of the combined cap. And the inner cap will contact directly with the medicine. the good quality of the inner cap can gives us a safety guarantee for our health.Our workshops comply strictly with the GMP standard and this allows to offer high quality products at best prices.
Pull-Ring Cap,Pull-Ring Cap For Plastic Bottle,Pull-Ring Cap For Soft Bag,Bottle With Pull-Ring Cap
Suzhou CRH New Material Technology Co.,Ltd. , https://www.crh-health.com