Recently, a woman named Liu Hongbin was famous. She traveled to Tibet, Qinghai, Inner Mongolia, Henan, Jilin, Heilongjiang, and Fujian provinces. She has a variety of important positions: Miao doctor, medical president, nutrition expert, health care physician, research committee...
Her countless avatars are serving a purpose: selling fake drugs.
"Don't look at today's jumping, be careful to pull the list tomorrow." After Liu’s experts and many of his colleagues were exposed, the iron fists of public opinion fell like rain.

At the end of the carnival, the crowd dissipated, and it seems that there is little concern about the fake drug advertisements on the TV. Now is the time to calm down and sort out the ins and outs: Why are fake TV advertisements on local TV stations so embarrassing?
The top spot for illegal advertising
The drugs that dare to appear on TV are subject to the approval of the State Food and Drug Administration. Since it is approved, where are these medicines?
On the one hand, the efficacy of counterfeit drugs in advertising is too magical, and the Nobel Prize in Medicine is Sweden’s eyes. Taking a three-month without a heart stent is too realistic. A week to say goodbye to gout and half a month to cure urine sugar is the standard configuration of the medicine.
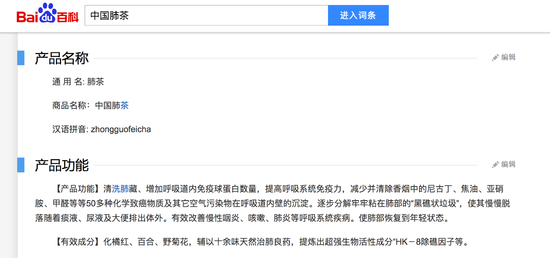
The medicines listed on an encyclopedia website: simple formula, curative effect / net
On the other hand, counterfeit drug advertisements are keen to invent concepts that are unheard of in the mainstream medical profession, such as the surrealist terminology of HK-8 except reef factors and reefs.
There are also many so-called drugs, which are essentially health products and cannot be counted as drugs. Health care products are profiteering industries, and many manufacturers who have not obtained production approvals are taking advantage of the opportunity to manufacture and sell health care products with the name of drugs. According to a survey released by the Shanghai Consumer Protection Committee: One in four people mistakenly used health products as medicines.
Kelly Health official website is full of cottage feeling, its GMP certification on CFDA query no results / screenshot
The United States Food and Drug Administration (FDA) has published the Dietary Supplement Health and Education Act (DSHEA), which provides for dietary supplements. A product (other than tobacco) intended to supplement the diet may contain one or more of the following dietary ingredients: A vitamin, a mineral, an herb (herbal) or other plant, an amino acid, a food component used to increase the total daily intake to supplement the diet, or a concentrate, metabolite, component, extract or combination of the above ingredients, etc. It also includes approved new drugs, vitamins or biologics that have been marketed as dietary supplements or food products before they are approved, issued or licensed. The DSHEA defines dietary supplements as their composition and labeling requirements: the product form may be pill, capsule, tablet or liquid; The product shall not be used as a substitute for ordinary foods or as an exclusive dietary item. The product shall be labeled as a "dietary supplement".
Dietary Supplements,Arachidonic Acid Powder,Kava Root Powder,Kava Root Extract Powder
Xi'an Double H Health Technology Co., Ltd , https://www.dhextract.com