What is osmotic pressure?
An osmometer is an efficient tool for determining the number of particles in blood, urine, or other fluids. The osmotic pressure will increase during dehydration, whereas when the water pressure is high, the osmotic pressure will decrease. There are three types of osmotic pressure: urine, plasma and stool. Urine osmotic pressure is the detection of renal function, blood osmotic pressure is a sign of the recruitment of blood diseases; stool test is the cause of diarrhea.
General application
The osmometer can be used as an effective and reliable drug screening tool. Similarly, osmometers can be used in forensic and criminal crime scenes as a tool for forensics. For example, teenagers who often go to clubs often add drugs to their drinks, and osmometers can easily detect whether drugs are added to beverages. An osmometer can also be used in environmental testing.
Â
The Vapro® 5600 Dew Point Osmometer is an instrument that uses the dew point method to determine the osmotic pressure of a sample. Compared with traditional instruments, the sample can be directly measured for high viscosity, samples containing suspended particles or multiple solvents without special treatment. In addition to ordinary liquid samples, the 5600 dew point osmometer can directly measure solid samples such as animal and plant tissue cell sections, and has a wide range of applications. 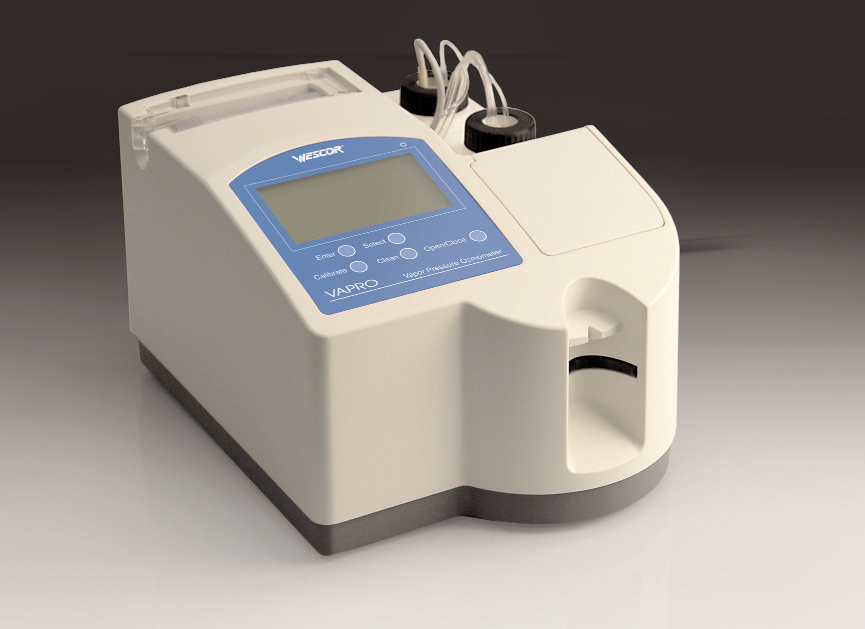 Â
 
Pharmaceutical Industry
1. What is muscle health?
a. Normal or slightly contracted state of healthy muscle fiber contraction.
b. The effective osmotic pressure of the solution.
In the parenteral solution of the drug, some tonics can be added to the hypnotic drug in the form of glucose.
2. Why are tonics important to pharmaceutical manufacturers?
The drug solution is usually a fine film that acts on the body and therefore must be the same as the nutrients in the body.
3. What is isostatic?
The isotonic solution does not cause tissue swelling or does not cause contraction when in contact with tissue. If it is in the eyes, nasal passages, or infused into other parts of the body, it will not cause discomfort.
4. What is low pressure?
The concentration of nutrients in the solution is less than the concentration in the cells. When a low pressure occurs, water enters the blood cell compartment causing the cells to swell and eventually breaks the cells. If the body cells can not withstand the above water pressure, it will cause water poisoning, resulting in muscle spasm or edema.
5. What is high pressure?
The nutrient concentration of the solution is greater than the cell concentration. This phenomenon can cause many complicated complications, such as hyperglycemia and dehydration.
6. What are the symptoms of red blood cell atrophy?
Water in the cell membrane attempts to dilute the concentration of salt around the cell membrane until the concentration of the erythrocyte membrane is the same inside and outside. The outflow of water causes the cells to shrink and wrinkle. In this case, the salt solution is high pressure.
Not all red blood cells are permeable, for example it is not a perfect semi-permeable membrane. In this way, it will allow not only the passage of water molecules, but also a mixture of macromolecules such as urea, alcohol, etc., through the red blood cells. The molecules of these components can pass through red blood cells and can cause hemolysis under any circumstances.
The mucus in the eye acts as a true semi-permeable membrane for boric acid, so boric acid can be used as an eye to balance nutrients.
7. Why do people suffer when eating high-osmotic osmotic pressure due to high electrolytes, amino acids and simple sugars?
When high osmotic nutrients are ingested, a large amount of water will be transferred to the stomach and intestines. The gastrointestinal tract contains a lot of water that can cause swelling, cramps, nausea, vomiting, and shock.
The body tries to maintain the osmotic pressure levels of the stomach and intestinal components as close as possible to the fluid surrounding them, but the sensitivity to osmotic pressure varies from person to person.
Bioengineering
1. Why are some cell culture media better than others?
The medium used for mammalian cell growth is a biological fluid such as plasma. This medium is subject to a number of adverse conditions, including batch changes and hazards such as contamination.
2. Why are other media better?
Reducing the composition of the medium is essential for cell growth. The discovery of basal media and its essential minimum components provides a wide range of uses and is currently commercially available.
3. What constitutes the medium?
The medium consists of a complex mixture of salts, sugars, amino acids, vitamins, hormones and growth factors. The concentration of salt is equal to the pressure to prevent the imbalance of penetration.
4. What are the components of the osmotic pressure test medium?
The standard for mammalian cell growth is an osmotic pressure of 300 m/osm, which is the best value for most cells.
5. How to make cell culture medium?
The main method of making cell culture until today is still the fermenter. Fermenters allow cells to grow and accumulate in vitro, just like growth in the body. The other is a rotating cell culture system that allows cells to accumulate because it provides a milder environment than a dynamic or static tissue.
Sports drugs
Frequently asked questions about hydration and dehydration
1. What is the definition of dehydration?
Lack of fluid demand for the body to work optimally. Dehydration is common due to vomiting, diarrhea or excessive urination. Dehydration between infants and children is most commonly caused by acute gastritis with vomiting and diarrhea.
Dehydration is divided into three levels: mild, medium or severe.
2. When can I cause dehydration during exercise?
The cause of dehydration is due to excessive sweating, including excessive fluid loss, inappropriate fluid inhalation, or a combination of these factors.
3. What are the signs of dehydration?
mild
Thirst, dry lips, dry mouth
Moderate
Very dry mouth film
Depression of the eye
Depression of baby skin
Skin depression
serious
Fast, weak pulse
Cold hands and feet
Breathing fast
Blue lips
Confused, burnout, sleepless
4. How to make up for lost liquid?
If there is not much fluid lost, athletes can drink beverages containing electrolytes and nutrients.
5. Why are sports drinks very popular?
When a physical exercise lasts for more than 60 minutes, sports drinks are good for them because they provide a source of fuel for the muscles and accelerate the absorption of water and glucose. Therefore, people who exercise for a long time should drink this type of drink. Due to the sporty drink, continuous exercise does not quickly deplete the hunger. And it can also quickly recover the lost strength.
6. When do you quote the drink?
Sports drinks are useful both before and after exercise. It can replenish lost body fluids, energy and minerals lost during exercise. Both the carbohydrate concentration and the salt concentration contribute.
7. How to drink this type of drink?
Some suggestions for drinking this type of drink:
Before exercise:
• Drink 8-24 ounces 2-4 hours before exercise
• Drink 4-8 ​​ounces before exercise
When exercising:
• Drink 4 ounces every 20 minutes after 30 minutes of exercise
After the exercise:
• 8-16 ounces
8. What is the difference between energy, hydration and restorative drinks?
Energy drinks contain a lot of sugar, which can reduce the hydration effect of the beverage.
2. Hydrated beverages do not contain sugars that can be replenished due to large amounts of glycogen loss.
Restorative beverages do not provide rapid energy absorption and hydration. The best restorative drink actually allows insulin release to stimulate the production of glycogen.
9. What is wrong with sports drinks?
If the sugar content of the beverage is high, the stomach will not consume it quickly. This will cause a lot of body fluids to flow back into the stomach, which in turn will lead to further hydration, and will not give you the benefits that these beverages can provide.
Stomach-intestinal disease
If the drink contains too much fructose, the stomach is difficult to digest and absorb it. An increase in the level of gastric acid can cause gastrointestinal diseases, and acid is contained in all non-alcoholic beverages for the purpose of taste and product stability. However, if the acid level in the beverage is too high, it often causes gastrointestinal diseases. And gastrointestinal diseases can also be caused by drinking the wrong drink at the wrong time.
Hypoglycemia
Hypoglycemia is a severe blood sugar level that takes away energy from the body. Hypoglycemia is the biggest problem with many sports drinks. Unless the beverage is continuously consumed during continuous exercise, the insulin response is somewhat reduced when exercised, and when the sports drink consisting of glucose or sugar breaks down into glycogen, it will cause an insulin reaction. Because the body's desire is to maintain a dynamic balance, insulin release will reduce blood sugar levels, and if blood sugar is too low, it will lead to low blood sugar. Insulin stores glucose in a hormonal manner. It prevents the concentration of sugar in the blood from rising and converts the excess sugar into fat or glycogen. This is why high carbohydrates cause obesity. It is important to control the secretion of insulin.
Insulin secretion is too low or not become diabetes. This will lead to many complications of the tissue, if there is more insulin secretion, or insulin is very effective, the decomposition of sugar will be very fast, leading to weight loss.
10. Is it possible to drink a lot of drinks?
Yes. Most runners and bikers know the severity of dehydration, but some people don't realize that excessive dehydration can dangerously lower the level of sodium in the blood – low blood sodium.
Hyponatremia is common in women, marathon runners, and triathletes for two reasons. 1, a lot of sweating lost a lot of sodium particles; 2, they are super sensitive to hydrates. He said: "They drink a lot of water before the big game, and then stop during the exercise."
Before the big game, if you drink a small amount of water, it is very likely to get tired in advance. But if you drink too much, there is a danger of low potassium.
11. How to avoid low potassium symptoms?
The key to avoiding low potassium is 1) Don't drink more water than you sweat 2) Drink salty sports drinks.
Stool osmotic pressure
Measurement of the osmolality of the stool can indicate whether the patient has diarrhea. In normal stools, most small molecules of matter can be completely absorbed (except electrolytes); therefore, many of the osmotic pressure of the stool comes from the electrolyte osmotic pressure. The difference in stool osmotic pressure refers to the difference between the measured osmotic pressure and the calculated osmotic pressure (the sum of sodium and potassium ions in the stool), or more precisely the serum osmotic pressure.
Osmotic diarrhea
• Unabsorbed ingredients
• Due to indigestion excrement
Secretory diarrhea
* Due to some food damage to the intestinal mucosa (burn, infection, etc.)
Most diarrhea is due to infection or bacterial toxins. Diarrhea is the main symptom of this phenomenon. Measurement of stool osmotic pressure does not make sense in this case. If diarrhea has lasted for more than a week, and the culture is negative, then diarrhea The reason is hard to find out. Gastrointestinal experts have pointed out two types of diarrhea: osmotic pressure type and secretory type. Osmotic pressure type diarrhea, there are some unabsorbed substances in the stool, which can prevent the normal absorption of water. At this time, the patient's osmotic pressure is 50 mOsm / kg. This type of patient is due to eating a lot of laxatives, it may also be eating some nutrients
If the internal mucosa of the gastrointestinal tract is damaged and cannot absorb water and electrolytes, then this is secretory diarrhea.
When measuring the osmotic pressure of the stool, it is necessary to consider the active substance produced by the metabolism of the bacteria, so the measurement must be completed within 30 minutes after the sample is collected, or the sample needs to be frozen.
Plant biotechnology
Hydration of plants
Plants balance their physiological metabolism by absorbing CO2, so they need to maintain adequate water storage inside. Photosynthesis occurs in the chloroplasts of plants, mainly in the cavernosal.
• There are many photosynthetic units in the sponge that capture photons.
• The energy exchange of chloroplasts is mainly to protect cells.
During the respiration of plants, the concentration of CO2 and water is dispersed in the mesophyll cells.
Tissue cells undergo photosynthesis to emit intercellular CO2 gas, which will result in a high concentration of CO2- in the air, flowing into the cell-low concentration. The bubbles of the mesophyll cells are surrounded by water - inside the texture, distributed in the protoplasts of the tissue cells. If the valve is closed, liquid moisture evaporates from the cell wall until the gas in the mesophyll cells is filled with saturated water vapor. The relative humidity of the ambient atmosphere is generally less than 100%, so if the valve is open, water vapor will be emitted into the atmosphere. The loss of water vapor in the mesophyll cells leads to further evaporation of water, which ultimately leads to a botanical "evaporation".
In order to survive, the root must absorb moisture from the outside. This can be done through the internal grain system of the plant. The lines of water-absorbing pipes are pipes that continuously absorb moisture and can carry water from the bottom to the top. 4. It is the pressure of the root that pushes the pressure.
• Root pressure comes from endothelial cells using transport proteins to concentrate internal substances. The high concentration of insoluble substances reduces the water inside the plants and must therefore absorb water from the root tip.
• The liquid is not compressible, so water entering from the bottom will increase the water pressure.
Another force that produces the above phenomenon is the "capillary phenomenon", which absorbs energy from the top. Capillary phenomena are mainly 1) because water has a high viscosity. 2) Capillary phenomena can absorb water into a narrow space if there is a chemical component such as cellulose dissolved in the water. This is why water can go up (as opposed to gravity).
The main concept of understanding capillary phenomena is surface tension.
• Surface tension comes from viscous forces: this force minimizes water to air contact;
• Water can also adhere to cellulose despite viscous forces;
• Evaporation of water reduces the cell wall between mesophyll cells.
If the gas enters the vessel, it will permanently cut off the water column. In this case, the breath will pull up and expand the bubble.
The water loss caused by breathing is mainly due to drought or dry climate. So when photosynthesis is not strong, you need to close the valve.
The change in the shape of the guard cell also means a change in the valve.
• The guard cells are cylindrical and their cell walls are tightly tied together.
• Guard cells are arranged in an orderly manner, and cell fibers can form an annular opening at the columnar edge.
The main signal that causes the guard cells to open the valve is the light intensity, which can stimulate the opening of the photon pump in the guard cell membrane.
The photon pump hydrolyzes ATP and ADP and uses the energy of this reaction to transfer H+.
• The increased negative pressure in the plasma membrane will cause positive ions to enter the cell, but only if the pump on the membrane is turned on and a large amount of K+ ions flow into the cell.
• Guard cells that receive signals exchange H+ and K+ ions efficiently. This will reduce the water inside the cells, and the water will re-inflow into the cells to compensate. Thereby changing the shape of the guard cells.
Question: How does the exchange of H+ and K+ cause a reduction in the risk of water? We already know that most solutions have the same effect as water, for example, for a 0.1 M solution, the water pressure is = -0.23. The key is that we must recognize that H+ is not a solution but a constituent element of water.
Excessive evaporation is not harmful to all plants and can be used as a temperature control function. Many enzymes and other proteins can only work within a limited range. Evaporation is a heat-dissipating process that cools the surfaces of solids and liquids and evaporates water into the air. Evaporation can cool a blade up to 10-15 ° C, thus protecting the enzymes during photosynthesis. This process is an automated mediation process.
Mammal tissue Â
Osmotic pressure: the key to improving tissue culture
In order to allow tissue cells to maintain their original morphology and function, we need to provide tissue growth conditions. The osmotic pressure of the medium is the main consideration.
For example, in humans, cells in tissues are surrounded by cytosol and other cells. When the cells are removed from the tissue culture and placed on a plastic or glass surface, a liquid envelop is still required. When placed in an external environment, if the osmotic pressure is different, it will cause osmotic shock.
Osmotic shock can cause changes in cell volume. Studies have shown that even small, short-term changes in cell volume can affect cell metabolism and gene expression. Therefore, we must pay attention to the impact of osmotic pressure on cells.
Measurement of osmotic pressure can increase tissue culture rate
Experience has shown that consciously measuring the osmotic pressure of the medium can effectively increase cell yield. Because the composition of the medium purchased each time varies greatly, it must be tested before use.
Since the density component of cell separation is always maintained in an isobaric range, measuring the osmotic pressure of the medium can significantly increase tissue culture. Moreover, maintaining normal osmotic pressure can maintain the activity of the enzyme and prevent the distortion of the membrane. But how to measure osmotic pressure can increase the success rate of cell culture?
Control osmotic pressure
Wyoming studies in 1970 showed that mammalian blood osmotic pressure and osmotic pressure of mammalian cell culture media are very important. The experiment was verified by many experimental results.
Study of kidney and bone marrow tissue using osmotic pressure
For example, cells in the kidney are often exposed to NACL and urea environments and must be able to adapt to ultra-high osmotic pressure environments. Some people
Maurice B. Burg, Natalia I. Dmitrieva, et al, did a lot of experiments to verify whether osmotic pressure affects cells and what mechanisms the cells produce to resist changes.
The results of Burg et al. showed that various levels of osmotic pressure were experienced both inside and outside the cell, and several protective mechanisms were used to accommodate high osmotic pressure.
Reaction to osmotic pressure
Because experiments have shown that environmental stress may mediate t-PA gene expression, EG Levin, L. Santell et al. did whether changes in osmotic pressure would affect the experiment of the gene. Even from an ultra-high osmotic environment to an isobaric environment, Hela cell culture showed increased T-Pa gene expression.
Experiments with recombinant CHO cells carrying the T-Pa gene under serum-free culture conditions were investigated. Cell expression can be confirmed to be affected by changing the osmotic pressure.
Moreover, changes in osmotic pressure can also alter the expression level of RNA, and many people have conducted experiments.
W. M. Miller, VM deZengotita et al. found that CO2 and osmotic pressure can affect the growth of hybrid cells in different media. But if the two are used together, cell growth will be suppressed to a large extent.
KC Katsirdakis mediates osmotic pressures in the range of 729 and 930 mOsmol/kg. When there is no light at 25-degree C, the pH is between 5.2 and 7.2, which can lead to the growth of grape leaves.
Ultra-high osmotic pressure
JE Olsen and GZ Li found that when high osmotic pressure is caused by sodium and potassium ions in cultured star cells, the cell membrane remains unchanged. They also found that sodium channels are activated at very low osmotic pressures and release large amounts of sodium and potassium ions.
Studies by GB Tennant's have shown that the growth of cloned bone marrow cells grows rapidly at low osmotic pressure, indicating that the osmotic pressure cells have great sensitivity and are strong for the growth of clonal cell lines.
Reaction caused by changes in osmotic pressure conditions
KB Marcum and CL Murdoch studied the salt tolerance of coastal plants by stimulating root growth with moderate salt concentrations, by constantly adjusting the osmotic pressure of salt concentrations up to 450 mM NaCl. They believed that protoplasts would change osmotic pressure at the highest salt concentration. .
Tissue cell grafting
The osmometer can provide information that cannot be provided in other ways, such as some useful laboratory parameters. Osmometers are critical in diagnosing patients in hospitals and can reduce treatment costs.
Osmometers are useful for measuring frozen point samples. Other methods are not sensitive to volatile materials and may be misleading in the diagnosis of diabetic patients.
manufacturing
The USP company in the United States requires measurement of the osmotic pressure of the injectable drug, but this value is not obtained by conventional methods.
BSR Murty, et al. tried to solve this problem, but initially the method was established only for solutions containing two components, which were later easily calculated using an osmometer.
USP wants to solve the osmotic pressure of its solution by osmometer! The osmometer is more accurate when measuring the isotonic point of a salt solution, but it is wrong to apply it to all solutions.
Pharmaceutical industry: Hospital mixes, drug manufacturers and nutritionists often use osmometers. This advantage can include detection consistency, minimizing trauma and optimizing reaction kinetics.
Eye medicine products
Ophthalmic products: By maintaining critical osmotic pressure parameters, it is possible to prevent morphological changes in the vitreous and infiltration into the eye solution.
Exercise drug
In exercise drugs, osmotic pressure is a versatile test that can improve the quality of a drug. Testing the hydration status of an athlete is the main use of the osmometer. Athletes often face dehydration and heat loss, especially at high temperatures and very hot weather. Measuring body weight before and after exercise can know how much water is being consumed. Drinking nutritious drinks, water and penetrating clothes can reduce athletes' chances of dehydration. The osmometer can be used to detect the urine sample of the athlete. It is also possible to measure the blood to obtain a more accurate indication. The osmotic pressure of a urine sample is an accurate indication of hydration changes and peak conditions.
Emergency medicine
The simplicity and practicality of the osmometer can be easily used in emergency ambulances to detect fluid therapy and surgical response. The freezing point method is the main way in which osmotic pressure is applied in this field because they are sensitive to many substances. The barometric pressure osmometer cannot read volatile gas components, thus losing useful information for toxicological testing.
Selection of non-toxic drugs: Drug substances, as a substitute for alcohol, are often used as new youth absorbents. Unlike alcoholic substances, this type of feeding can be detected by an osmometer.
Head loss: A cell pressure-reducing substance of mannitol can sometimes cause acute encephalitis quickly. Experiments have shown that mannitol can enter the brain from the blood, eventually leading to changes in osmotic pressure. The osmotic pressure can be quickly measured using a freezing point osmometer.
Detection of mannitol treatment: The detection of the concentration of the osmotic active ingredient is a major advantage of the freezing point osmometer. In mannitol, neurobiologists use osmotic active ingredients to control brain edema. The aim is to maintain an increase in osmotic pressure of 10 mOsm/Kg, although an increase in speed of 5 mOsm may be more effective.
Maintaining a small increase in osmotic pressure is important because too high a blood osmotic pressure appears to increase blood pressure and cerebral blood pressure between cells. If the osmotic pressure difference reaches 50 mOsm/Kg, it may be nephritis. Mannitol treatment of elevated intercellular pressure can treat children with Reye. The blood pressure is measured 12 times a day to ensure that the blood pressure does not exceed 340 mOsm/Kg. Of course, volatile mannitol cannot be measured by a vapor osmometer.
Coma: The patient shows an unresponsive or messy mental state, and blood osmotic pressure is a tool for effective evaluation of mental state.
Burns: Osmotic pressure provides a quick analysis of the dehydration status of burn patients and can detect correct fluid therapy.
Quality Control: Pharmaceutical, hospital and nutritionists can use osmometers to detect treatment consistency and minimize trauma.
Emergency medications: The simplicity of the osmometer can be easily used in emergency ambulances to detect fluid therapy and surgical response.
Alcohol-free test: Drinking alcohol will increase blood osmotic pressure by 23 mOsm per 0.1%.
Toxicological differences: Using osmotic pressure instruments we can detect some small molecule toxic solutions, methanol, ethanol and mercury are the most common emergency housing things.
Internal medicine
High osmotic pressure: The actual osmotic pressure is the true osmotic pressure. Urine osmotic pressure helps determine if osmotic pressure is budding.
Inappropriate ADH Symptoms: Blood and urine osmolality can help determine if SIADH is present in the blood of a normal patient.
ADH treatment: Urine osmotic pressure can be used to quickly measure a patient's response to treatment.
Diabetes Treatment: Bird osmotic pressure can measure the effects of hormonal factors and provide rapid disease diagnosis.
Insulin therapy: The osmometer provides a quick assessment of insulin treatment.
Early warning patients:
Osmotic pressure helps assess renal function, fluid balance and electrolyte balance.
Embryology: Osmotic pressure is an important tool to ensure the bonding of eggs and embryos, especially during the first few days of culture.
Surgical treatment
Ultra-high/ultra-low osmotic shock therapy: The osmometer can be used to detect coronary osmotic pressure and avoid excessive osmotic shock symptoms.
Electrode or physiological fluid imbalance: Osmotic pressure provides rapid postoperative electrode fluid or physiological fluid imbalance testing. In urologic surgery, the difference between the calculated osmotic pressure and the measured osmotic pressure can be used to indicate the liquid flow absorption.
Intravenous therapy: The osmometer can be helpful when administered intravenously.
Liver transplantation: Changes in osmotic pressure are often caused by loss of blood or blood transfusion.
Glycine Amino Acid Uptake: Prolonged surgery can result in a large amount of fluid input, which ultimately leads to an increase in osmotic pressure.
Urology and nephrology
Decreased renal function: The osmotic pressure of urine and serum has a great influence on the moisture reaction inside: water loss, dilution, etc.
Uremic: The difference in osmotic pressure after measurement and calculation can indicate the accumulation of uncontaminated waste in the blood. Once the basic electrolyte level is established, osmotic pressure can be used to detect osmotic pressure in uremic patients.
Osmotic pressure: The difference in osmotic pressure can be calculated in urine and blood, and can provide early pathological tips.
Renal dialysis: In patients with kidney disease, if the osmotic pressure of the blood rises, dialysis is required. Dialysis osmotic pressure can detect the process during treatment.
Renal function: The osmometer is an essential tool for measuring blood and urine. It can detect polyuria, ADH and insulin.
Patients with normal blood volumes, measuring their osmotic pressure can help establish a SIADH diagnosis. The osmometer can provide rapid results when using ADH to diagnose superosmotic pressure. The difference in free water can be quickly measured to provide early symptoms. An osmometer can help distinguish between patients with diabetes and tissue lesions.
The osmotic pressure cannot be directly indicated by the data provided by the automatic electrolyte analyzer. Instead, more information must be identified by the osmotic pressure difference. (The dew point osmometer article was transferred from China Biomaterials Network)
Dew point osmometer: http://
phone
Fiber Glass Panel Security Door
Fiber Glass Panel Security Door,Fiberglass Security Door,Fiberglass Panel For Security Door,Italian Fiberglass Security Door
Zhejiang Tilanco Industry&Trade Group Co., Ltd , https://www.tilancogroup.com